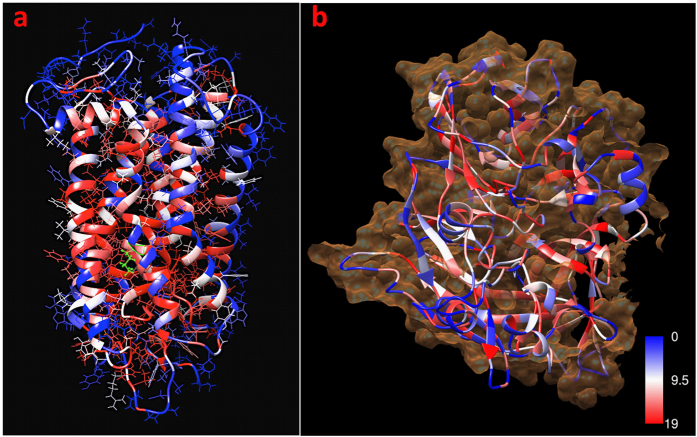
Figure 2. Foldability coloring for the rhodopsin and RPE65 protein structures.
The red residues represent the wild-type residues that exhibit the most severe unfolding effects (high foldability) when mutated, while the blue residues maintain their stability (low foldability) when mutated. (a) Side chains of rhodopsin residues with the active site K296 highlighted in green to show the critical residues of the rhodopsin structure surrounding the active site. (b) The surface of RPE65 is shown in orange around the ribbon structure, revealing severe unfolding within the β-sheets. The foldability coloring was obtained in the program Chimera using the Python script described in the Methods section.