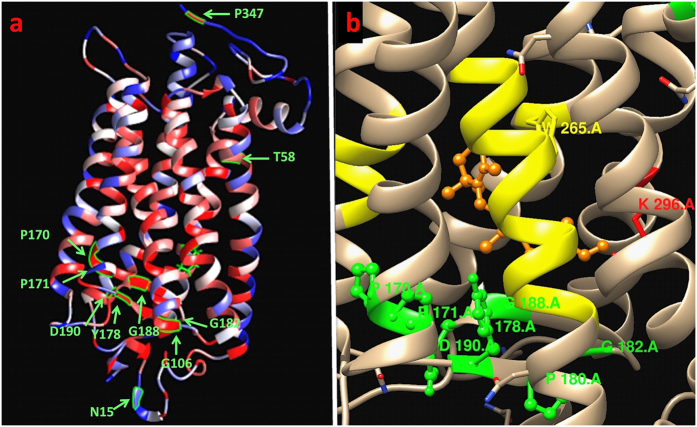
Figure 3. Foldability structure of rhodopsin displaying the mutations from Table 2.
(a) The amino acid residues affected by the mutations are shown in green. All mutations except N15S have high foldability, indicating residues that are essential for proper protein folding. (b) The majority of mutations with high foldability values lie near the retinal chromophore binding site. The retinal binding residues and N6-(retinylidene) lysine (K296) are yellow and red, respectively. The retinal molecule is shown in orange.