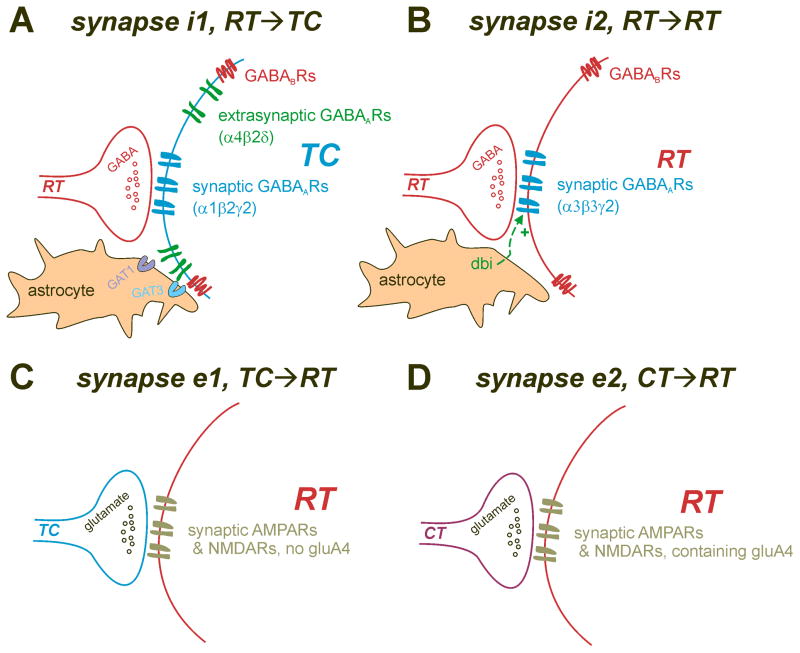
Figure 3. Synaptic elements in thalamic circuit that regulate synchrony and oscillations.
A. RT →TC, synapse i1. This inhibitory synapse is responsible for the phasic inhibition that drives post-inhibitory rebound firing in TC cells. GABA is released to activate α1β2γ2 GABAARs within the synapse, and can spillover to activate extrasynaptic α4β2δ GABAARs and GABAB receptors with slower kinetics. Spillover to extrasynaptic receptors is tightly regulated by GABA uptake via astrocytic GAT1 and GAT3. B. RT →RT, synapse i2. Chemical inhibitory signaling between RT cells is largely dependent on α3β3γ2 GABAARs, although a weak GABAB component is also present. Astrocytes in RT appear to release an endogenous benzodiazepine site ligand derived from benzodiazepine binding inhibitor (DBI) producing a constitutive positive allosteric modulation of RT GABAARs, that suppresses synchrony in the network. C. RT →TC, synapse e1. This synapse provides excitatory feedback within the thalamic loop to reinforce spindle oscillations and SWD. Although gluA4 is the major AMPA receptor subunit in RT, it does not appear to contribute to excitation at this synapse, as it’s synaptic strength is unchanged by deletion of gria4, which encodes gluA4. D. CT →RT, synapse e2. This synapse provides feedback from the cortex that can reinforce global CTC oscillations. Inactivation of gria4 weakens this synapse, leading to underexcitation of RT, and failure to produce cortical feed-forward inhibition (synapse i1) with resultant hyperexcitation at the CT→TC synapse, e3 (not shown).