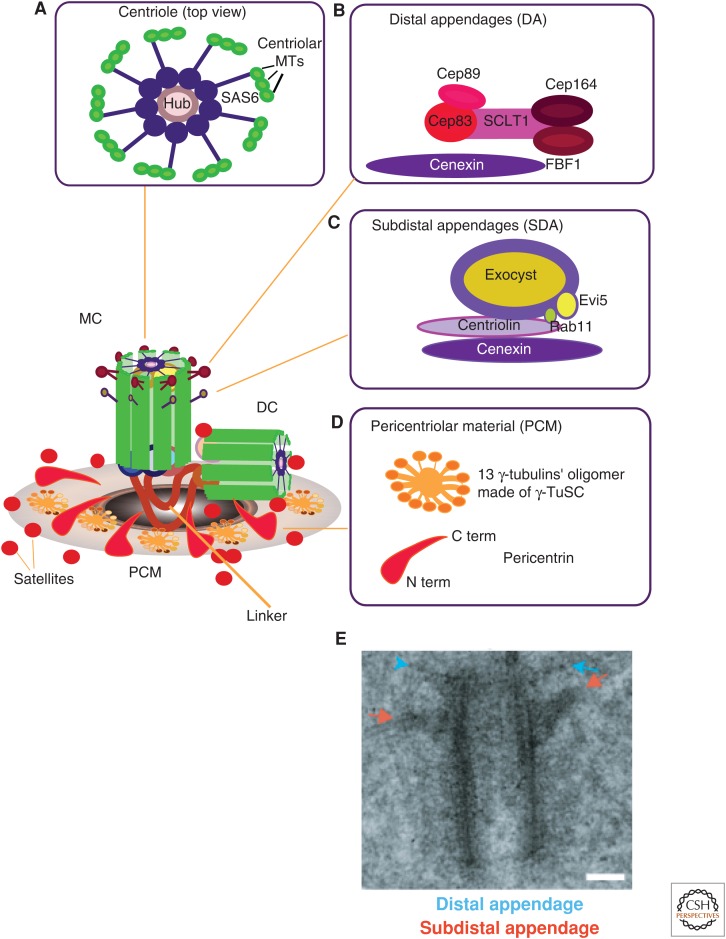
Figure 1.
Centrosome substructures. A model highlighting the centrosome and its specific substructures, namely, (A) the centrioles, (B) distal appendages, (C) subdistal appendages, and (D) pericentriolar material (PCM). The centrosome comprises the mother centriole (MC), daughter centriole (DC), tethered together by “linker” structures, PCM, and satellites. The “linker” ensures centriole engagement and timely linker degradation that licenses centriole duplication during S phase. The protein composition of the “linker” remains elusive, but the involvement of pericentrin, Cep215, and Cep68 in centriole connections has recently been shown (Pagan et al. 2015). (A) Illustration showing the centriole barrel (in top view). Depicted is a central hub, a rod-shaped structure of SAS6 homodimers that form oligomers (Kitagawa et al. 2011; Guichard et al. 2013), from which nine spokes of SAS6 homodimers emanate, which each radiate toward a microtubule (MT) triplet. (B) Relative organization of molecular players forming the distal appendages (DAs), with Cep83 being in closest proximity to the centriole. (C) A model in which it is proposed that cenexin/Odf2 is responsible for the integrity of the subdistal appendages (SDAs) and for connecting them to the MC. (D) Organization of the PCM into 13 protofilament oligomers that contain γ-tubulin in the outermost layer; the carboxyl terminus (C term) of pericentrin is located close to the centriole barrel, whereas the amino terminus (A term) is oriented toward the outer lattice layer. (E) Electron micrograph of a mother centriole with two sets of appendages; DAs are highlighted by blue arrows and SDAs are highlighted by red arrows. Scale bar, 0.1 µm. (E, Reproduced from Hung et al. 2016, with permission from Elsevier.)