Figure 12.
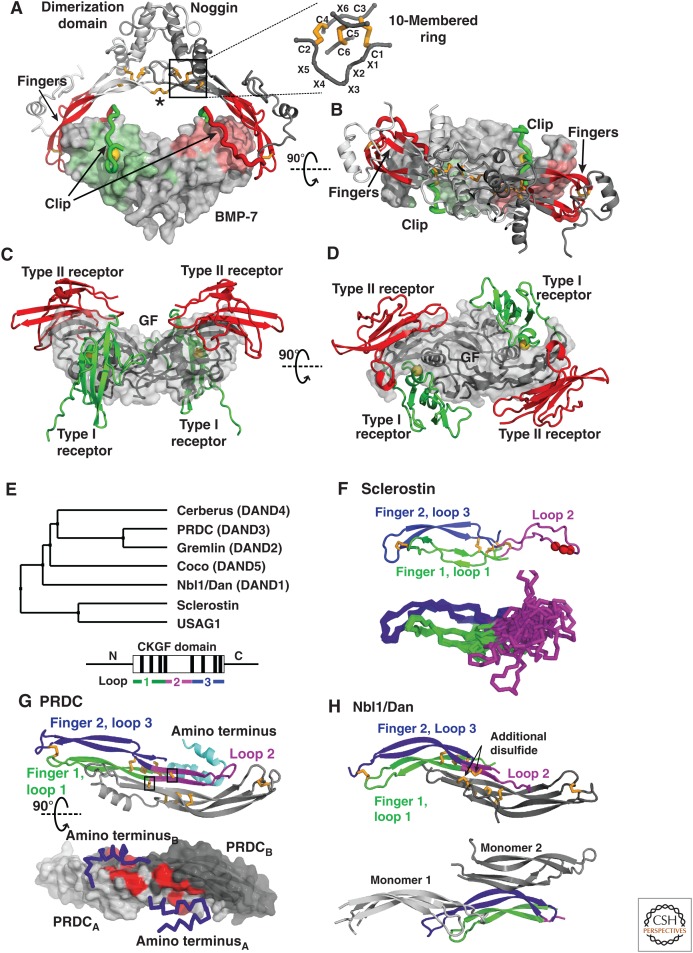
The bone morphogenetic protein (BMP) modulator noggin, members of the Dan family, and sclerostin. (A) Noggin binds BMPs (PDB 1M4U [Groppe et al. 2002]) in a clamp-like manner with the fingers of its cystine knot (CK) growth factor (CKGF) domain (red) blocking type II receptor binding, and a peptide segment termed clip (green) shielding the type I receptor epitopes. Hydrophobic Pro35 of noggin, engaging in the knob-into-hole interaction motif is shown as a gold sphere. The noggin dimer is formed by interactions between the α-helical dimerization domains and stabilized by an intermolecular disulfide bond (asterisk). Disulfides are shown with gold sticks and the CK motif (box) is shown in detail. (B) As in A but viewed down the dyad axis of the noggin and GF dimers. (C and D) A prototypical BMP ligand-receptor complex, with type I and type II receptors as green and red ribbons, respectively, and the GF dimer as gray surface (PDB 2H64 [Weber et al. 2007]) shown in similar orientations as in A and B. (E) Phylogenetic tree of the Dan family (upper panel) and schematic of domain organization (lower panel) with the CKGF domain boxed, its eight disulfides indicated by black lines, and the segments forming three loops colored as in panels F-H. The highly diverse segments amino- and carboxyl termini to the CKGF domain are shown as horizontal lines. (F) Sclerostin NMR structure (PDB 2KD3 [Weidauer et al. 2009]). The cartoon representation (upper) shows the NXI motif (red spheres) and the Cα atom trace (lower) shows the ensemble of 15 structures highlighting the flexibility of loop 2. Loops are colored as in E (lower). (G) PRDC (PDB 4JPH [Nolan et al. 2013]). The cartoon representation (upper, with one monomer colored as in E, lower) shows cysteines as gold sticks, with the unpaired Cys boxed. The lower panel shows PRDC as a surface, with the α-helical amino-terminal segments as blue Cα atom traces. Hydrophobic residues, which on exchange for alanine attenuate binding to BMP binding, are colored red. (H) Two Nbl1/Dan crystal structures (PDB 4X1J [Nolan et al. 2015], upper) and (PDB 4YU8, Table 1, lower). One monomer is colored as in E (lower). The other monomer(s) associates as in PRDC (gray, upper) or in a different manner, with two nearby monomers in the crystal lattice shown in two shades of gray (lower).