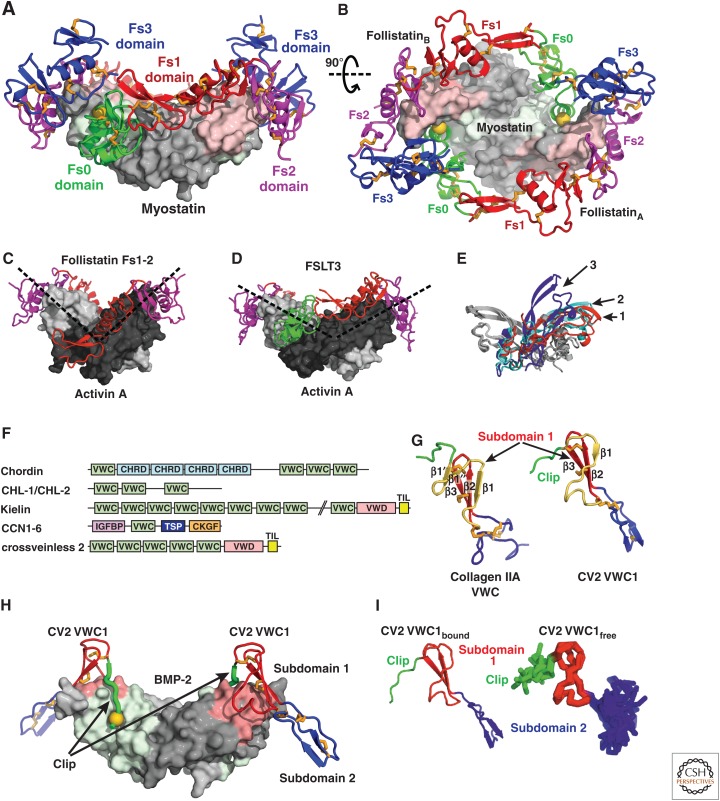
Figure 13.
Modulators of the follistatin and chordin families. (A,B) Two follistatin-288 molecules shown in ribbon cartoon wrap around myostatin shown as a surface (PDB 3HH2 [Cash et al. 2009]). Fs0 (green) blocks access to the type I receptor epitope (light green) with a knob-into-hole interaction indicated by a gold sphere; Fs1 (red) and Fs2 (magenta) block the type II receptor-binding site (pink). (C–E) Open and closed conformations of activin A. (C,D) Shows activin monomers as light and dark gray surfaces in different orientations (dashed lines) in an open complex with an Fs1-2 fragment of follistatin (C, PDB 2ARP [Harrington et al. 2006]) and in a closed conformation with FSTL3 (D, PDB 3B4V [Stamler et al. 2008]). (E) Compares monomer–monomer orientations in activin A and myostatin dimers by superimposing on one monomer (gray) and showing the orientation of the other monomer (colored and numbered). The colored and numbered monomers are from dimeric structures of myostatin bound to follistatin-288 (red, 1), activin A bound to FSTL3 (cyan, 2) and activin-A bound to the follistatin Fs1-2 fragment (blue, 3). (F) Chordin family members are mosaics of tandem domains. Although von Willebrand factor C (VWC) domain(s) mediate bone morphogenetic protein (BMP) binding, other domains present include chordin (CHRD), von Willebrand type D (VWD), trypsin inhibitor-like (TIL), insulin-like growth factor binding protein (IGFBP), thrombospondin (TSP) and cystine knot growth factor (CKGF). (G) VWC domain of collagen IIA (left) and VWC domain 1 of CV2 (right). Amino-terminal peptide segments and VWC subdomains 1 and 2 are green, red and blue, respectively. A structurally variable segment of VWC subdomain 1 is shown in yellow. (H) The VWC1 domain of CV2 shown in ribbon with knob-into-hole residue shown as gold sphere bound to BMP-2 shown as surface with type I and type II receptor sites colored green and pink, respectively (PDB 3BK3 [Zhang et al. 2008]). (I) Similarity in architecture of VWC1 domain of CV2 bound to BMP-2 in crystals (left) and free in solution (right panel, NMR ensemble of 10 structures, PDB 2MBK [Fiebig et al. 2013]).