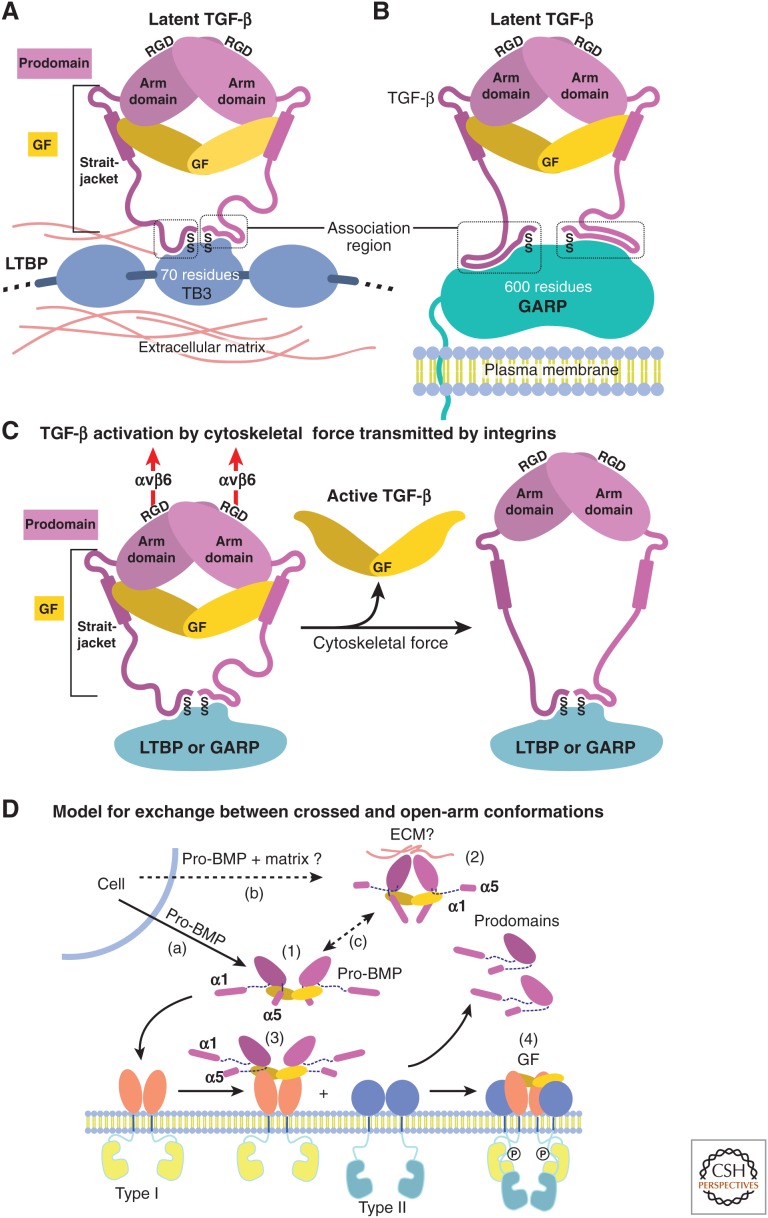
Figure 3.
Models for growth factor (GF) latency, activation, and binding to receptors. (A,B) Schematic representation of how anchoring molecules present pro-transforming growth factor β1 (TGF-β1) in the extracellular matrix (ECM) or on cell surfaces, and how the association region at the amino terminus of the prodomain must take on different conformations to bind distinct anchoring molecules and two different sites within each anchor molecule. (C) Schematic representation of pro-TGF-β1 activation (Shi et al. 2011). Integrins, such as αvβ6, attached to the actin cytoskeleton exert traction force against RGDLXXL/I motifs in pro-TGF-β1 and -β3, which are covalently linked to anchor molecules in the ECM or on the cell surface. The most force-susceptible structural region is the straitjacket and its latency lasso, which will be elongated. Consequently, TGF-β1 is released, and shifts conformation to its free form. (D) Models for conformational change between crossed-arm, latent, and open-arm nonlatent conformations of pro-BMPs (Mi et al. 2015). (1) Open-arm, nonlatent conformation; (2) putative crossed-arm conformation of pro-BMPs stabilized by association with ECM components; (3,4) step-wise binding to type I (3) and type II (4) receptors. Putative pathways for secretion (a,b) and conformational interconversion (c) are marked.