Figure 1. Signalling pathways underlying the go pathways.
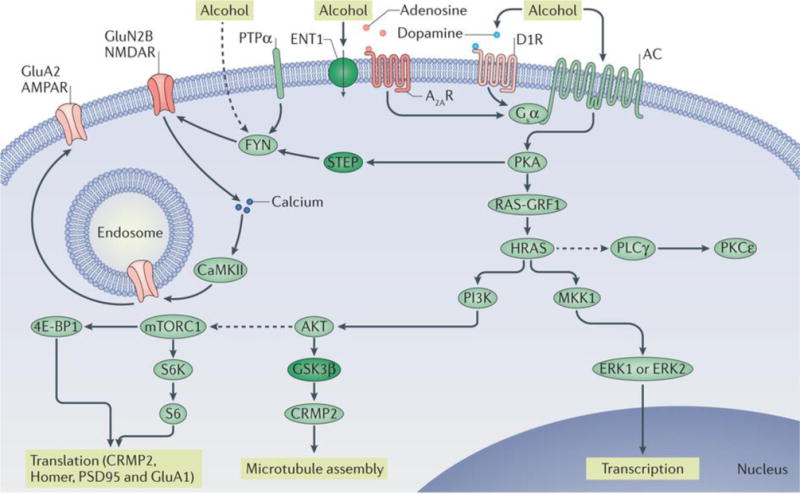
Repeated cycles of excessive alcohol exposure and withdrawal lead to aberrant activation of specific intracellular signalling cascades (the go pathways), the nature of which varies depending on the brain region. Collectively, these alcohol-related changes in intracellular signalling cascades drive long-lasting, detrimental behavioural phenotypes associated with alcohol abuse, such as excessive consumption, alcohol seeking, craving and relapse. Protein kinase A (PKA) has a central role in the go pathways. PKA is activated by adenylyl cyclase (AC), which hydrolyses ATP to cyclic AMP and is activated by alcohol through several mechanisms, including the inhibition of equilibrative nucleoside transporter 1 (ENT1) (inactivated proteins are shown in dark green) and subsequent activation of the Gsα-coupled adenosine A2A receptors (A2ARs) and/or the activation of the Gsα-coupled dopamine D1 receptor (D1R). cAMP binds to the regulatory subunit of PKA, thus freeing the catalytic subunit of the kinase to phosphorylate its substrates, which include striatum-enriched protein-tyrosine phosphatase (STEP). Activation of FYN requires the recruitment of protein-tyrosine phosphatase-α (PTPα), which dephosphorylates an inhibitory phosphorylation site. STEP is inactivated by PKA phosphorylation, which enables the sustained activation of FYN. Alcohol might also activate FYN by additional mechanisms (as indicated by the dashed line). When FYN is activated, it phosphorylates the GluN2B subunit of NMDA-type glutamate receptors (NMDARs), resulting in enhancement of NMDAR activity. Calcium entry via the NMDARs activates calcium/calmodulin-dependent protein kinase type II (CaMKII), resulting in autophosphorylation of the kinase. CaMKII phosphorylates the AMPA-type glutamate receptor (AMPAR) subunit GluA2, resulting in forward trafficking of these receptors to the synaptic membrane. Another target of PKA is RAS-specific guanine nucleotide-releasing factor 1 (RAS-GRF1), which, when activated, promotes the transition of the small GTP-binding proteins HRAS and KRAS from inactive GDP-bound forms to active GTP-bound forms. Activation of HRAS leads to the activation of phosphoinositide 3-kinase (PI3K). PI3K, in turn, activates AKT, which phosphorylates glycogen synthase kinase 3β (GSK3β), thus inhibiting its activity. When GSK3β activity is reduced, collapsin response mediator protein 2 (CRMP2) binds to tubulin, enabling microtubule assembly. AKT, through intermediate proteins, also activates mechanistic target of rapamycin complex 1 (mTORC1) (as indicated by the dashed line). mTORC1 phosphorylates its substrates eukaryotic translation initiation factor 4E-binding protein 1 (4E-BP1) and p70 S6 kinase (S6K), which then phosphorylates its substrate S6. 4E-BP1, S6K and S6 are part of the ribosomal translational machinery, and activation of mTORC1 initiates the translation of postsynaptic density protein 95 (PSD95), Homer, CRMP2 and GluA1. HRAS also activates mitogen-activated protein kinase kinase 1 (MKK1), which in turn activates extracellular signal-regulated kinases 1 and 2 (ERK1/2), inducing gene transcription. Moreover, HRAS indirectly activates (as indicated by the dashed line) phospholipase Cγ (PLCγ), which in turn activates protein kinase Cε (PKCε).
