Abstract
In the present study, porous silica particles as well as impervious fused-silica wafers and capillary tubes were modified with hydrophilic polymers (hydroxylated polyacrylamides and polyacrylates), using a surface-confined grafting procedure based on atom transfer radical polymerization (ATRP) which was also surface-initiated from α-bromoisobutyryl groups. Initiator immobilization was achieved by hydrosilylation of allyl alcohol on hydride silica followed by esterification of the resulting propanol-bonded surface with α-bromoisobutyryl bromide. Elemental analysis, IR and NMR spectroscopies on silica micro-particles, atomic force microscopy, ellipsometry and profilometry on fused-silica wafers, as well as CE on fused-silica tubes were used to characterize the chemically modified silica substrate at different stages. We studied the effect of monomer concentration as well as cross-linker on the ability of the polymer film to reduce electroosmosis and to prevent protein adsorption (i. e., its non-fouling capabilities) and found that the former was rather insensitive to both parameters. Surface deactivation towards adsorption was somewhat more susceptible to monomer concentration and appeared also to be favored by a low concentration of the cross-linker. The results show that hydrophilic polyacrylamide and polyacrylate coatings of controlled thickness can be prepared by ATRP under very mild polymerization conditions (aqueous solvent, room temperature and short reaction times) and that the coated capillary tubes exhibit high efficiencies for protein separations (0.3–0.6 million theoretical plates per meter) as well as long-term hydrolytic stability under the inherently harsh conditions of capillary isoelectric focusing. Additionally, there was no adsorption of lysozyme on the coated surface as indicated by a complete recovery of the basic enzyme. Furthermore, since polymerization is confined to the inner capillary surface, simple precautions (e.g., solution filtration) during the surface modification process are sufficient to prevent capillary clogging.
Keywords: ATRP grafting from, hydride silica, hydrosilylation, hydroxylated monomers, hydrolytic stability
Graphical Abstract

1. Introduction
Fused-silica capillary tubes used in GC and micro-bore LC have also found extensive use in the modern version of electrophoresis, CE(1, 2). In contrast with chromatography, chemical modification of the fused silica capillary has been aimed primarily at eliminating unwanted interactions between the inner wall of the CE tube and the sample undergoing separation. Many chemical modifications schemes used to modify silica-based chromatographic substrates have been readily extended to electrophoretic capillaries (3–5). Strictly speaking, the type and number of active surface functionalities on silica surfaces (silanols, siloxanes, etc.) depend on factors such as synthesis method, thermal history and the presence of humidity. Additionally, the irregular porous structure of silica micro-particles governs the accessibility of reagents to the active silica sites during surface modification. This kinetic factor aside, the chemistries of porous and flat silicas are essentially equivalent. It is also accepted that wafer surfaces are geometrically and chemically equivalent to the inner surface of fused-silica capillaries; they are both flat at the molecular level. Surface coverage resulting from organic groups grafted on a flat silica surface should be significantly denser than that of the same groups on a curved surface (particulate porous silica) since the later is sterically more constrained resulting in dissimilar group conformations (6).
One of the greatest challenges in the current practice of CE is the requirement of surface deactivation of capillaries toward protein adsorption. Such unsolved bottleneck arises from the nonspecific interactions (ion exchange, hydrogen bonding, dispersion, etc.) between the inner capillary wall and the solute. Such unwanted interactions are responsible for excessive peak tailing, incomplete solute recovery and unreliable quantification. As a result, the high speed, separation efficiency, selectivity and versatility, minimal sample size requirement and easy automation of CE cannot be fully exploited in the case of many proteins. Researchers have suggested that a polymeric film that furnishes a hydrophilic, stable and adsorption-resistant (non-fouling) barrier between the capillary wall and the solute is required in protein analysis (7, 8). Such materials are also of great interest in many other fields such as medical sciences, biosensors, contact lenses technology, enzyme-based immuno assays, to name just a few (9).
The attachment of polymers to solid surfaces via chemical bonding is an important strategy to modify the substrate properties in such a manner that the nonspecific interactions between the protein molecules and silica surface are minimized (7, 10). Anchoring of a polymer onto a silica surface has been achieved by two main strategies, the “grafting-to” and the “graftingfrom” methods. The grafting-to approach involves the attachment of a prefabricated polymer from solution, via the formation of a covalent bond between polymer active groups and matching groups on the substrate surface. Water-soluble polymers such as poly(ethylene glycol) and poly(vinyl alcohol) are typical examples of preformed polymers used in the grafting-to technique. Hydroxyl groups of the polymer react with surface-immobilized active species, such as glycidyl (11). Despite its experimental simplicity, the grafting-to strategy is limited by strong steric hindrance effects that worsen with increasing polymer size and eventually hinder contact of incoming polymer with surface reactive sites (10). In the graftingfrom approach, propagating polymer chains grow from surface-immobilized initiator groups. Among the various procedures for the grafting-from method, atom transfer radical polymerization (ATRP) is especially attractive for its remarkable control over the molar mass of the grafted polymer, great versatility (works well with a variety of functionalized monomers), compatibility with water and the possibility of mild polymerization temperatures (12–14). In the ATRP-based grafting-from approach, chain transfer and thermal self-initiation processes are essentially negligible and polymeric chains grow exclusively from the surface; i.e., the process is both surface-initiated and surface-confined. The initiator moieties are most usually anchored to the silica surface via silane coupling chemistry. Halogenated ATRP initiator groups, such as benzyl chloride or, to a much greater extent, 2-bromo-isobutanoyl have been used in the past to grow neutral hydrophilic films on fused silica capillaries. Wirth and her research group reported the first covalent bonding of a polymer film for CE by ATRP in 1998 (15). Surprisingly, very few papers have been published about ATRP applied to CE since then (16–18).
Although acrylamide has been the most commonly used monomer to make polymeric coatings for CE (4, 7), the limited stability at moderately high pH of polyacrylamide has been a well-known fact from traditional slab gel electrophoresis (19). The slow deamidation of the polymer under this condition leads to a considerable deterioration of the coating, evidenced by the formation of fully-dissociated carboxylic groups that cause strong electroosmosis, polymeric layer swelling and analyte band distortion. The use of N-substituted acrylamide derivatives such as N-acryloylamino-ethoxyethanol, has resulted in coated capillaries with superior resistance to hydrolysis and hence improved long-term CE separations at alkaline pH (20). N-acryloyl-aminoethanol (AAE) (21–23) and its relative N-acryloyl-aminopropanol (AAP) (24, 25), also N-substituted acrylamide derivatives, should provide durable coatings as well. It appears that the N-substitution with hydroxyl-terminated chains produces polymeric coatings that are not only hydrolytically more stable, but have also higher hydrophilicity compared to polyacrylamide (19, 24, 25). When it comes to electrophoretic performance, hydrophilicity turns out to be of paramount importance since a high hydrophilicity of the polymer effectively precludes proteins to compete with water for its potentially adsorptive sites (26). It has been suggested that any monomer less hydrophilic than acrylamide should be considered unsuitable to produce a good quality gel “since acrylamide is itself already at the border-line between hydrophilicity and hydrophobicity” (25).
There is a definite need for hydrophilic coatings of improved hydrolytic stability that enable the use of CE at its full potential, and such improved materials are frequently targeted at protein separations. In the present work we explore the combination of several promising synthetic schemes to modify the fused-silica surface of capillaries for a lasting resistance to protein adsorption. More specifically, our work attempts to bring together the best of three worlds: (i) a stable anchorage of the polymerization initiating group to the inner wall of the capillary tube by means of Si–C linkages formed by hydrosilylation; (ii) a stable polymeric film whose strength arises from the N-substitution on acrylamide; and (iii) a surface-confined in-situ polymerization method (ATRP) that prevents clogging of the capillary tube while permitting an easy control of the coating thickness.
2. Materials and Methods
2.1 Instrumentation
IR spectroscopy was performed on a Shimadzu, Model FTIR-8400 spectrometer (Columbia, MD, U.S.) equipped with a diffuse reflectance infrared Fourier transform (DRIFT) accessory. Solid state NMR characterization was carried out using a Bruker Advance II-400 MHz NMR spectrometer (Rheinstetten, Germany) equipped with a Bruker MAS II probe. Electrophoretic separations were performed on Agilent model 7100 or 1600 CE Systems (Palo Alto, CA, U.S.). Atomic force microscopy (AFM) images were obtained with an Asylum Research model MFP-3D-SA (Santa Barbara, CA, U.S.) instrument. Carbon percent measurements were carried out on a FlashEA 1112 elemental analyzer Thermo Inc. (Waltham, MA, U.S.).
2.2 Materials
Fused-silica capillaries (50-μm id, 360-μm od and 20-μm polyimide coating) were purchased from Biotaq Inc. (Silver Springs, MD, U.S.) and Polymicro Technologies (Phoenix, AZ, U.S.). UV-grade fused-silica wafers (25.4 × 9.0 × 1.0 mm) were purchased from Laser Optex Inc. (Beijin, China). Silica micro-particles (Nucleosil, 7-μm diameter and 91.0-m2 g−1 surface area, and YMC Gel, 10-μm diameter and 288-m2 g−1 surface area) were obtained from Macherey-Nagel (Duren, Germany) and YMC Co. (Kioto, Japan) respectively. Piperazine-N, N′-bis(3-propanesulfonic acid) (PIPPS) was purchased from GFS Chemicals (Columbus, OH, U.S.). Nacryloyl-aminoethanol, N-acryloyl-aminopropanol, 2-hydroxyethyl methacrylate, α-bromoisobutyryl bromide, tris[2-(dimethylamino)ethyl]amine (Me6TREN), bipyridine (bpy), allyl alcohol, platinum(0)-1,3-divinyl-1,1,3,3-tetramethyldisiloxane (Karstedt’s catalyst, ~2 % Pt in xylene), hexachloroplatinic acid, cuprous chloride, 2,5-di-tert-butylhydroquinone, potassium cyanide, EDTA, 3-(N-morpholino)propanesulfonic acid (MOPS), methylcellulose, Ampholytes pH 3-10 and pH 6–8 (40% aqueous solutions) and DMSO were purchased from Sigma-Aldrich (St. Louis, MO, U.S.). Analytical grade solvents were obtained from various vendors; they were freshly distilled from sodium shavings before use.
2.3 Surface modification
Prior to modification, fused-silica capillaries (typically, a 6.0-m length) and wafers were conditioned by etching with NaOH followed by leaching with HCl, according to procedures described elsewhere (27). Porous silica particles did not undergo any conditioning. Hydride silica substrates were prepared as described previously (28). 3-hydroxypropyl (propanol) bonded silica phase was prepared by hydrosilylation of allyl alcohol on hydride silica, as described in a recent report (29). Esterification of the bound propanol groups was carried out by treatment for 6 h at room temperature (20 ± 2 °C) with a solution containing 0.25 M of α-bromoisobutyryl bromide and 0.25 M of pyridine in dry DMF under a nitrogen blanket (30–32). This solution was prepared in a nitrogen-purged glove bag. The product was sequentially rinsed with 10%v/v water in DMF and THF, and then dried under nitrogen. Polymerization of 2-hydroxyethyl acrylate (HEA), AAE and AAP was catalyzed with CuI(M6TREN)+Cl− in 4:1 v/v ethanol/water solvent (23). 2-Hydroxyethyl methacrylate (HEMA) was polymerized with CuI(bpy)2+Cl− in 1:1 v/v methanol/water solvent (33). For a typical polymerization in a capillary, a solution containing 1.0 M monomer and 20 mM Cu(I) catalyst in alcohol/water solvent was passed through the column for 3 h at room temperature (18, 30). This solution was prepared with helium-degassed solvent in a nitrogen-purged glove bag. At the reaction end, the column was sequentially rinsed with solvent and water for 30 min each. The column was emptied with nitrogen gas for a few minutes. Fused-silica wafers were modified by immersion in the reagent solution with gentle shaking, while 65 mg of porous silica were used per mL of reagent solution with magnetic stirring.
2.4 Spectroscopy and microscopy
DRIFT, solid state NMR and AFM measurements were obtained accordingly to previously described procedures (28). An Uvisel 2 Spectroscopic Phase Modulated ellipsometer was used for film thickness measurements at Horiba Scientific laboratories (France). Perfilometry measurements were carried out with a KLA Tencor D-120 stylus profiler (U.S.) at the Materials Engineering School of Universidad del Valle.
2.5 Surface coverage
The concentration, ΓR, of surface-bonded R-groups on porous silica was obtained from elemental analysis of the bonded material along with the BET specific surface area of the hydride intermediate substrate (34).
2.6 CE experiments
Prior to use, capillaries were conditioned with the electrolyte (typically, 25 mM PIPPS, adjusted to pH 4 with NaOH) under high pressure (7 bar) for 20 min, after which a stable electroosmotic mobility (EOM) value was obtained (27). EOM determinations followed methodology previously described in the literature (35, 36). CIEF separation conditions were adapted from a method devised by Hempe et al. (37, 38).
3. Results and discussion
3.1 Silica substrate modification
The synthetic scheme used to chemically modify the inner capillary surface with a polymeric film is outlined in Figure 1. Initiator immobilization is accomplished by hydrosilylation of allyl alcohol on hydride silica followed by esterification of the resulting propanol-bonded surface with α-bromoisobutyryl bromide. The first step is based on the formation of a stable Si–C bond by the catalytic addition of silicon hydride to an olefin (hydrosilylation). We have recently demonstrated that allyl alcohol can also be anchored to the hydride silica surface and that it does so with a high coverage (3–5 μmol m−2) (29). In the esterification of the anchored alcohol groups with α-bromoisobutyryl bromide, we found that using DMF as solvent and pyridine as HBr-binding base maximize the solubilization of the bromide salts produced during the esterification process. This is important to minimize clogging of the tube as the reaction is performed on fused-silica capillaries. When this two-step procedure is applied on porous silica micro-particles, ligand density information from the primary (hydrosilylation) as well as the secondary (esterification) modifications can be readily estimated from percent carbon contents along with specific surface area and structural information (molar mass and number of carbon atoms) of the anchored groups (34). Our results indicate that a typical propanol-modified substrate with a surface coverage of 3.5 μmol m−2 (2.1 chain nm−2) produced an α-bromoisobutyryl coverage of 1.1 μmol m−2 (0.75 chain nm−2), which means that about 30% of the surface propanol groups were esterified. One can think of the resultant functionalized silica surface as consisting of active α-bromoisobutyryl initiator moieties surrounded by a dense layer of inactive propanol groups. Naturally, denser surface coverages of the α-bromoisobutyryl groups should be expected on a flat silica surface in comparison to the same groups on a curved surface such as that of porous silica. Since the average cross sectional area of a polymer chain (~1.8–2.0 nm2) is much larger than that of a typical initiator group (~0.20 nm2) (39, 40), our relatively low α-bromoisobutyryl coverage (1.5 nm2 chain−1) should provide the footprint surface area required for a dense polymer grafting quite well, as described below. Previous studies show that such modest α-bromoisobutyryl group density favors an efficient polymer growth (41).
Figure 1.

Schematic diagram illustrating the hydrosilylation of allyl alcohol on the Si–H surface, followed by esterification of the propanol-functionalized silica surface to immobilize the α-bromoisobutyryl initiator, and then by surface-initiated and -confined polymerization of N-acryloyl-aminopropanol via ATRP.
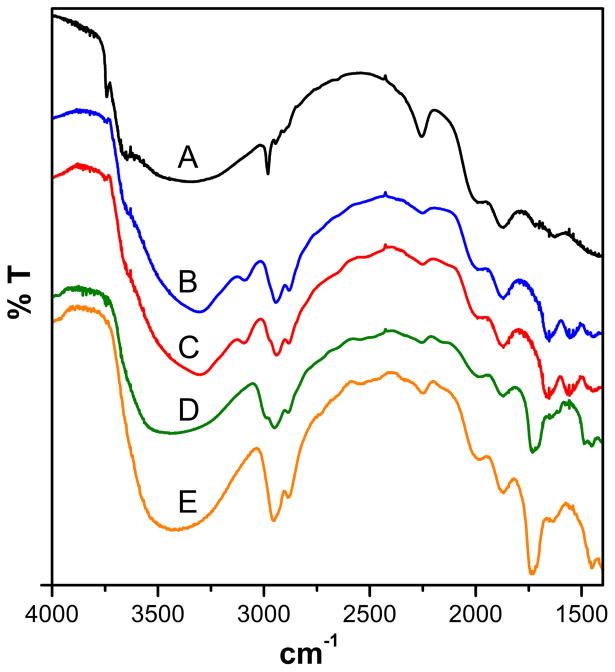
When we applied our ATRP-based grafting-from approach to several hydroxylated monomers–namely, HEA, HEMA, AAE, and AAP– efficient polymerization took place. Monomer structures are depicted in Figure S-1 of the Supplementary Material. It is important to point out that we used mild conditions for the polymerization reaction: aqueous solvent, room temperature and short reaction times, as suggested by Armes (31, 33) and Kakuchi (23). These conditions represent a significant departure from those proposed by Wirth in her original report (100°C, dry DMF solvent, and 40-h reaction time) (15). Undoubtedly, the milder conditions are more attractive while still favoring good grafting density of the polymer. Furthermore, we used a much more common and (about 500 times) more active α-bromoisobutyryl group as initiator. Figure 2 shows IR spectroscopic evidence that confirms polymerization from the immobilized α-bromoisobutyryl initiating groups. A substantial increase of stretching bands in the 2950–2850 cm−1 region is observed, which is indicative of symmetric and asymmetric aliphatic C-H stretching, along with the appearance of characteristic C=O stretching bands at 1655 and 1730 cm−1 for amides (curves B and C) and esters (curves D and E) respectively. Most likely, the bands at 3085 and 1550 cm−1 (curves B and C) can be assigned to the amido N–H stretching and bending modes respectively. Furthermore, 13C CP-MAS NMR spectra clearly verifies chemical bonding of the polymeric film to the silica surface, as shown in Figures S-2 to S-4 of the Supplementary Material. To further characterize the polymeric film on the silica surface, AFM was used on coated silica plates by probing the surface after polymerization. As shown in Figure 3, the surface is uniformly coated with the polymeric film and exhibits a more rugose surface topography (rugosity = 3.16 nm), in comparison to an unmodified plate (~ 0.3 nm)(28). Thickness measurements with ellipsometry revealed a uniform film with a layer thickness of 53 ± 2 nm. Additionally, profilometry measurements provide a thicknesses of 59 ± 8 nm, which is quite consistent with that from ellipsometry. One intrinsic feature of ATRP-based grafting-from process is that the polymerization is confined to the substrate surface. Since free initiator is absent in the monomer/catalyst mix, no polymer is formed in the bulk of the solution, avoiding blockage of the capillary column. Additionally, a simple rinsing procedure provides adequate film cleanup. These details are of critical importance in the preparation of chemically modified CE tubes.
Figure 2.
DRIFT spectra of porous silica particles (Nucleosil) functionalized with (A) α-bromoisobutyryl initiator, and the polymerization products of (B) AAP, (C) AAE, (D) HEA and (E) HEMA monomers. Monomer concentrations, 1.0 M; catalyst concentrations, 20.0 mM; temperature, 25°C; reaction time, 3 h. Catalyst and solvent (B) through (D), [CuI(Me6TREN)]+Cl− and ethanol/water 4:1 v/v, (E), [CuI(bpy)2]+Cl− and methanol/water 1:1 v/v.
Figure 3.
Typical AFM image of a silica plate modified with a polyAAE film. The plate as probed in the intermittent noncontact mode with a force constant of 42 N/m and resonance frequency of 300 kHz. A rectangular silicon cantilever with tetrahedral tip and nominal spring constant of 0.06 N/m was used. The scan size was 3 μm × 3 μm. Preparation conditions as described in Figure 2.
3.2 Electrophoretic performance
Chemical modification of CE tubes is accompanied by a drastic drop of electroosmosis, the electrokinetic bulk flow of electrolyte originated at the wall/solution boundary. We evaluated the effect of monomer concentration on the ability of the polymer film to reduce electroosmosis and it was found to be very small, as expected, and essentially insensitive to variations of monomer concentration. This is shown in the Supplementary Material for polyAAE, polyHEA and polyHEMA coatings (Figure S-5). We next examined the CE performance of polymer-coated capillary tubes with respect to their ability to prevent protein adsorption (i. e., its non-fouling capabilities). Peak asymmetry and separation efficiency of two strong silanol-sensitive compounds, Ru(bpy)32+ and lysozyme, were used as indicators of the extent of adsorption on the coated capillaries upon varying monomer concentration. The Ru-complex is very sensitive to electrostatic interactions with silica surfaces, while lysozyme is also sensitive to other nonspecific interactions such as hydrogen bonding, hydrophobic effects, etc. The effect of increasing monomer concentration is evident in the case of polyAAE (1–2 M-levels appear to favor low asymmetry values and high plate count) and essentially absent in the case of polyHEMA (This is shown in Figure S-6 of the Supplementary Material). In the case of polyHEA, a concentration ≥ 2 M appears to favor high plate counts. Similar observations, albeit to a lesser extent, apply to the Ru probe (not shown). We also examined the effect of cross-linker concentration on polymer film behavior (chemical structures of the cross-linkers used are also depicted in Figure S-1 of the Supplementary Material) and found that, similarly to monomer concentration, there is no evident effect on EOM (see Figure S-7 of the Supplementary Material). On the other hand, low concentration of cross-linker (0.1% molar) appear to furnish lower asymmetry and higher plate count in the case of polyAAE, while there was very little or slightly detrimental effect for polyHEA and polyHEMA (see Figure S-8 of the Supplementary Material). Results are summarized in Figure 4, where it appears that, generally speaking, the presence of cross-linker improves the symmetry, separation efficiency and time-corrected area of lysozyme when the amide monomers, AAE and AAP, are used. In contrast, when the two ester monomers, HEA and HEMA, are used, the presence of cross-linker appears to be somewhat detrimental to the electrophoretic performance of the basic enzyme. It should be pointed out that there does not seem to be a most favorable monomer among the ones studied. PolyAAE exhibits the largest corrected peak area, which suggests maximum solute recovery. Cross-linked polyAAP shows the greatest separation efficiency, while uncross-linked HEMA has a remarkably low peak asymmetry.
Figure 4.
Comparison of lysozyme CE peak features on several polymeric coatings with and without their corresponding cross-linkers. Error bars represent ± 1 SD (n = 3). ATRP conditions equivalent to those in Figure 2. Cross-linker concentration, 0.1% molar with respect to the monomer. CE conditions: capillary, 35.0 cm (effective length, 26.5 cm); electric field, 400 V cm−1 (9.8 μA); electrolyte, 25.0 mM PIPPS buffer with pH 4.0; protein concentration, 0.5 mg mL−1 each in buffer; injection, 3 s at 50 mbar; detection, 210 nm.
Several proteins with high pI were separated to test the capability of the polymeric coating to resist adsorption. Figure 5 shows a typical electropherogram for a mixture of four of these proteins (cytochrome c, lysozyme, trypsin and ribonuclease A) at pH 4.0, with separation efficiencies comparable to those of former reports (15). Whereas separation efficiencies are very high (typically 0.25 to 0.65 million plates per meter; see Table S-1 of the Supplementary Material), CE performance at its full potential –a few million plates per meter– seems to be elusive, and some residual fouling appears to be an inevitable limitation of our hydrophilic coatings at this stage of development. The graphical abstract shows another example of the great selectivity obtained for two cytochromes C on a polyAAE-Bis modified capillary under the same CE conditions used in Figure 5. To further complement our observations, such residual adsorption can be quantitatively assessed by analyte recovery measurements. This is accomplished by linear fitting of logarithmic peak area vs. effective length data (42). For a given tube id the slope, k, is proportional to the surface density of adsorption sites and, hence, is a useful figure to comparatively assess protein adsorption on different capillary coatings. Preliminary experiments, summarized in Table 1, show that the k-values for both polyHEMA and polyAAP coatings are statistically undistinguishable from zero which, in turn, indicates a complete (100%) recovery of the basic enzyme; i.e., there is no adsorption on the coated surface. In contrast, the oligoamine (tetraethylenepentamine) used to dynamically coat a native capillary, while quite effective to reduce lysozyme adsorption under the same CE conditions, exhibits some measurable analyte lost during its passage through the capillary tube as indicated by a k-value which is statistically different from zero. With regards to precision, while the repeatability of migration times was 0.2-0.7 %RSD, the typical peak area precision was 5-13 %RSD (n = 10 successive injections). The later is likely due to the difficulties associated with the various isoforms that accompany the enzyme samples, as illustrated in Figure 5.
Figure 5.
CE separation of a mixture of basic proteins in a polyAAE-coated capillary. Conditions: capillary, 35.0cm (effective length, 26.5 cm); electric field, 400 V cm−1 (8.1 μA); electrolyte, 25.0 mM PIPPS buffer with pH 4.0; protein concentration, 0.1 mg mL−1 each in 10-fold diluted buffer; injection, 15 s at 20 mbar; detection at 200 nm. Solutes: (a) cytochrome C, equine, (b) lysozyme, chicken, (c) trypsin, bovine, (d) α-chymotrypsinogen A, porcine.
Table 1.
CE recovery of lysozyme as a function of the nature of the coating grafted on the inner wall of the capillary (42).
| coating | k, cm−1 a | sk | %Recovery b (l=26.5 cm) | s%recovery |
|---|---|---|---|---|
| polyHEMA c | 0.0015 | 0.0092 | 96 | 23 |
| polyAAP d | 0.0029 | 0.0044 | 93 | 11 |
| none (native) e | 0.0055 | 0.0018 | 86 | 4 |
Estimated from linear fitting of the logarithm of lysozyme peak area (referred to the internal standard) vs. effective length, l.
Estimated from the equation R = exp(-kl) with R as the analytical recovery of lysozyme and 26.5 cm being the minimum possible l in our instrument.
Uncross-linked. Other preparation conditions as described in Figure 2.
Cross-linked with 0.10% molar Bis with respect to the monomer.
Native tube etched with 1.0 M NaOH, leached with 1:1 v/v HCl and conditioned with electrolyte.
CE conditions: electrolyte, 25.0 mM PIPPS buffer adjusted to pH 4.00 ± 0.05 with 12.5 mM γ-aminobutyric acid; also containing 1.5 mM tetraethylenepentamine in the case of the native capillary tube. Sample: 1.0 mg mL−1 lysozyme and 3.0 mM histidine as internal standard in electrolyte (10.0 mM benzylamine as internal standard with the native capillary tube). Applied field, 400 V cm−1 (current, 5.8 μA). Five replicate injections (5 s at 0.67 mbar cm−1) were performed on a 50-μm capillary tube with varying effective length.
3.3 Long-term hydrolytic stability
We have chosen the very demanding electrolyte conditions of capillary isoelectric focusing (CIEF) to assess long-term hydrolytic stability of our polymeric coatings. While we used a hydrolytically durable N-substituted amide as the starting monomer along with a stable Si–C link to the silica surface, when a sodium hydroxide solution is used as cathode electrolyte –as it is done in CIEF, the underlying siloxane bridges that hold the silica backbone will eventually collapse. Although to a lesser extent, the phosphoric acid solution used as anode electrolyte will also be very aggressive towards the polymeric coating. Figure 6 shows typical CIEF separation profiles of blood samples on a polyAAP-Bis coated capillary tube. The two upper panels of Figure 7 illustrate measured migration time and area for two hemoglobin (Hb) peaks of a hemolyzed blood sample mix as a function of injection number during a reiterating CIEF experiment. Within the experimental error of this several-day-long experiment, peak migration time and, to a lesser extent, corrected peak area do not seem to deteriorate significantly. It should be pointed out that there is a certain trend associated with each 10-injection cycle (we renewed the sample after 10 consecutive injections) which contributes to the overall variability and may mask the long-term profile of the parameter under study. This effect of sample renewal is more pronounced for the peak area (mid panel of Figure 7) and for polyAAE coatings (not shown). Long-term precision for Hb S and A0 was about 5 and 23 %RSD for migration times and time-corrected peak areas respectively (n = 260). Regression analysis on the data (see Table S-2 of the Supplementary Material) reveals nearly zero slopes along with low correlation coefficients. The very small slope of, for instance, migration times vs. run number indicates that it would take an average of 15 and 45 runs to decrease by one second the migration times of Hb S and A0 respectively. Figure S-11 of the Supplementary Material visually corroborates the fact that the Hb separation profile does not deteriorate significantly after many sample injections. Perhaps more important from a stability point of view, the hydrolytic deterioration of a CE coating ultimately leads to an increased exposure of ionizable silanol groups and, hence, greater EOM. The impact of a prolonged exposure of a coated capillary to the harsh conditions prevailing in CIEF should provide an “ageing” curve that assesses its hydrolytic stability. Indeed, the EOM appears to be more revealing with regard to the effect of coating exposure to the harsh conditions prevailing in CIEF (see lower panel of Figure 7): precision of EOM stars declining after 200 runs and its value definitely increases above 260 runs. Yet, acceptable band profiles are obtained even after 270 runs, as depicted in Figure S-11 of the Supplementary Material. Unfortunately, comparable ageing experiments are not easy to find in the CIEF literature probably because these tests are expensive in terms of effort and time.
Figure 6.
Hemoglobin constituents in normal blood (left), and blood from a patient with sickle cell disease by CIEF. Whole blood (10 μL) was treated with 200 μL of hemolyzing solution (EDTA, 5 mM; KCN, 10 mM), and then introduced into the capillary by low-pressure injection (30 mbar for 17 sec). Cathode and anode solutions were 20 mM NaOH and 100 mM H3PO4 in 0.375% methylcellulose, respectively. Prior to each assay, the capillary was flushed (5 min under 0.95 bar) with electrolyte containing 0.375% methylcellulose and 2.5% v/v Ampholyte mix solution (pH 6–8 / pH 3–10, 10/1 v/v). Sample constituents were focused for 4 min at 25 kV, then eluted past the detector (UV absorbance at 415 nm) under simultaneous low pressure (20 mbar) and same voltage. Capillary: Bis-crosslinked polyAAP coating, 50-μm id and 35.0-cm length (26.5-cm effective length).
Figure 7.
Long-term stability test under typical CIEF conditions for a Bis-crosslinked polyAAP coating immobilized on a 50-μm id capillary with 35.0-cm length (26.5-cm effective length). Experimental CIEF conditions were the same described in Figure 6, except that the sample was a 1:1 v/v mixture of hemolysates from same normal and abnormal blood samples. 10 consecutive injections per sample aliquot were carried out from the same hemolysis lot. Electroosmotic mobility (EOM) was measured after every 10 CIEF runs. CE conditions for EOM measurements: hydrodynamic injections (3 s at 50 mbar) of 10 mM DMSO (neutral marker) in the electrolyte solution (25-mM MOPS buffer, pH 7.0), 28.0 kV voltage applied (18 μA) for 5.0 min, hydrodynamic mobilization at 50 mbar, detection at 215 nm, 1 min electrolyte rinse between runs. Error bars represent ± 1 SD (n = 3).
4. Conclusions and future perspectives
In this report, we describe surface-initiated and -confined ATRP methodology for chemically modifying silica surfaces with low-fouling polymer coatings. The results indicate that hydroxylated polyacrylamides and polyacrylates coatings of controlled thickness can be prepared by ATRP under very mild polymerization conditions (aqueous solvent, room temperature and short reaction times) and that the coated capillary tubes exhibit high performance for protein separations along with remarkable hydrolytic stability which affords favorable long-term reproducibility. Since polymerization is confined to the inner capillary surface, clogging of the narrow tube conduit becomes very unlikely if simple precautions are taken during the surface modification process. The controlled polymerization method can be easily extended not only to other hydrophilic monomers such as N-acryloylamino-ethoxyethanol (20, 43), but also to betain-related (e.g., sulfobetaineacrylamide) monomers that are known to form zwitterionic polymer surfaces that exhibit high resistance to nonspecific protein adsorption (44). These families of N-substituted acrylamide monomers should provide a step ahead towards the solution to the need for non-adsorbing coatings that enable the use of CE at its full potential, particularly for protein separations.
Although capable of efficiently initiating a variety of polymer chains, immobilization of the α-bromoisobutyryl group did not occur with the high yields expected from hydrosilylation and esterification (see Figure 1). Clearly, further work is needed to achieve a better control of silica surface coverage. There is also a concern regarding the relatively poor stability of the ester functionality at the opposite end of the chain containing the α-bromoisobutyryl initiator group (see the final structure of the sequence depicted in Figure 1). Such labile ester group is in clear contrast with the highly stable Si–C bond (formed by hydrosilylation) which fastens the propyl chain to the inner capillary wall, and with the polymeric film itself –a stable N-substituted polyacrylamide. It is also very important to simplify the synthetic scheme where immobilization of the initiator α-bromoisobutyryl group takes two steps. Our early attempts to anchor initiator groups to the hydride silica via the direct hydrosilylation of 4-vinylbenzyl chloride as well as allyl α-bromoisobutyrate (both commercially available) were unsuccessful, as described in the last section of the Supplementary Material. We are currently working on a new procedure to anchor the α-bromoisobutyryl initiator by means of a single-step reaction, whose results will be the subject of another report.
Supplementary Material
Highlights.
A controlled radical polymerization scheme is devised for stable, non-fouling CE coatings.
Polymers are surface-initiated from α-bromoisobutyryl groups under mild conditions.
High separation efficiency and complete solute recovery are obtained for lysozyme.
Clog-free capillaries inherently result from the surface-confined grafting method.
Acknowledgments
This work was supported by the National Institute of General Medical Sciences of the National Institutes of Health [grant number R01GM089759], and Universidad del Valle [project number CI-7832]. The content is solely the responsibility of the authors and does not necessarily represent the official views of the supporters.
Abbreviations
- EOM
electroosmotic mobility
- ATRP
atom transfer radical polymerization
- AFM
atomic force microscopy
- AAE
N-acryloyl-aminoethanol
- AAP
N-acryloyl-aminopropanol
- HEMA
2-hydroxyethyl methacrylate
- HEA
2-hydroxyethyl acrylate
- Me6TREN
tris[2-(dimethylamino)ethyl]amine
- bpy
bipyridine
- PIPPS
piperazine-N, N′-bis(3-propanesulfonic acid)
- CIEF
capillary isoelectric focusing
- MOPS
3-(N-morpholino)propanesulfonic acid
- Hb
hemoglobin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jorgenson JW, Lukacs KD. High-resolution separations based on electrophoresis and electroosmosis. Journal of Chromatography A. 1981;218:209–216. [Google Scholar]
- 2.Hjerten S. High-performance electrophoresis: the electrophoretic counterpart of highperformance liquid chromatography. Journal of Chromatography A. 1983;270:1–6. [Google Scholar]
- 3.Buchmeiser MR. New synthetic ways for the preparation of high-performance liquid chromatography supports. Journal of Chromatography A. 2001;918:233–266. doi: 10.1016/s0021-9673(00)00129-1. [DOI] [PubMed] [Google Scholar]
- 4.Doherty EAS, Meagher RJ, Albarghouthi MN, Barron AE. Microchannel wall coatings for protein separations by capillary and chip electrophoresis. ELECTROPHORESIS. 2003;24:34–54. doi: 10.1002/elps.200390029. [DOI] [PubMed] [Google Scholar]
- 5.Pallandre A, de Lambert B, Attia R, Jonas AM, Viovy J-L. Surface treatment and characterization: Perspectives to electrophoresis and lab-on-chips. ELECTROPHORESIS. 2006;27:584–610. doi: 10.1002/elps.200500761. [DOI] [PubMed] [Google Scholar]
- 6.Unger KK. Porous silica: Its properties and use as support in column liquid chromatography. In: Unger KK, editor. Journal of Chromatography Library. Vol. 16 Elsevier; 1979. [Google Scholar]
- 7.Horvath J, Dolnik V. Polymer wall coatings for capillary electrophoresis. ELECTROPHORESIS. 2001;22:644–655. doi: 10.1002/1522-2683(200102)22:4<644::AID-ELPS644>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 8.El Rassi Z. Electrophoretic and electrochromatographic separation of proteins in capillaries: An update covering 2007–2009. ELECTROPHORESIS. 2010;31:174–191. doi: 10.1002/elps.200900576. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee I, Pangule RC, Kane RS. Antifouling coatings: Recent developments in the design of surfaces that prevent fouling by proteins, bacteria, and marine organisms. Advanced Materials. 2011;23:690–718. doi: 10.1002/adma.201001215. [DOI] [PubMed] [Google Scholar]
- 10.Zhao B, Brittain WJ. Polymer brushes: surface-immobilized macromolecules. Progress in Polymer Science. 2000;25:677–710. [Google Scholar]
- 11.Zhao Z, Malik A, Lee ML. Adsorption on polymer-coated fused-silica capillary electrophoresis columns using selected protein and peptide standards. Analytical Chemistry. 1993;65:2747–2752. doi: 10.1021/ac00068a008. [DOI] [PubMed] [Google Scholar]
- 12.Matyjaszewski K, Xia J. Atom Transfer Radical Polymerization. Chemical Reviews. 2001;101:2921–2990. doi: 10.1021/cr940534g. [DOI] [PubMed] [Google Scholar]
- 13.Barbey R, et al. Polymer Brushes via Surface-Initiated Controlled Radical Polymerization: Synthesis, Characterization, Properties, and Applications. Chemical Reviews. 2009;109:5437–5527. doi: 10.1021/cr900045a. [DOI] [PubMed] [Google Scholar]
- 14.Matyjaszewski K. Atom Transfer Radical Polymerization (ATRP): Current Status and Future Perspectives. Macromolecules. 2012;45:4015–4039. [Google Scholar]
- 15.Huang X, Doneski LJ, Wirth MJ. Surface-Confined Living Radical Polymerization for Coatings in Capillary Electrophoresis. Analytical Chemistry. 1998;70:4023–4029. doi: 10.1021/ac980231c. [DOI] [PubMed] [Google Scholar]
- 16.Huang X, Wirth MJ. Surface Initiation of Living Radical Polymerization for Growth of Tethered Chains of Low Polydispersity. Macromolecules. 1999;32:1694–1696. [Google Scholar]
- 17.Feldmann A, Clausnitzer U, Otto M. Optimization of capillary coating by hydroxyethyl methacrylate for capillary zone electrophoresis of proteins. Journal of Chromatography B. 2004;803:149–157. doi: 10.1016/j.jchromb.2003.09.007. [DOI] [PubMed] [Google Scholar]
- 18.Miller MD, Baker GL, Bruening ML. Polymer-brush stationary phases for opentubular capillary electrochromatography. Journal of Chromatography A. 2004;1044:323–330. doi: 10.1016/j.chroma.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 19.Righetti PG, Gelfi C. Electrophoresis gel media: the state of the art. Journal of Chromatography B: Biomedical Sciences and Applications. 1997;699:63–75. doi: 10.1016/s0378-4347(96)00207-1. [DOI] [PubMed] [Google Scholar]
- 20.Chiari M, Nesi M, Sandoval JE, Pesek JJ. Capillary electrophoretic separation of proteins using stable, hydrophilic poly(acryloylaminoethoxyethanol)-coated columns. Journal of Chromatography A. 1995;717:1–13. [Google Scholar]
- 21.Albarghouthi MN, Stein TM, Barron AE. Poly-N-hydroxyethylacrylamide as a novel, adsorbed coating for protein separation by capillary electrophoresis. ELECTROPHORESIS. 2003;24:1166–1175. doi: 10.1002/elps.200390150. [DOI] [PubMed] [Google Scholar]
- 22.Zhao C, Zheng J. Synthesis and Characterization of Poly(N-hydroxyethylacrylamide) for Long-Term Antifouling Ability. Biomacromolecules. 2011;12:4071–4079. doi: 10.1021/bm2011455. [DOI] [PubMed] [Google Scholar]
- 23.Narumi A, et al. Poly(N-hydroxyethylacrylamide) Prepared by Atom Transfer Radical Polymerization as a Nonionic, Water-Soluble, and Hydrolysis-Resistant Polymer and/or Segment of Block Copolymer with a Well-Defined Molecular Weight. Macromolecular Chemistry and Physics. 2009;210:349–358. [Google Scholar]
- 24.Simo-Alfonso E, Gelfi C, Sebastiano R, Citterio A, Righetti PG. Novel acrylamido monomers with higher hydrophilicity and improved hydrolytic stability: I. Synthetic route and product characterization. ELECTROPHORESIS. 1996;17:723–731. doi: 10.1002/elps.1150170418. [DOI] [PubMed] [Google Scholar]
- 25.Simo-Alfonso E, Gelfi C, Sebastiano R, Citterio A, Righetti PG. Novel acrylamido monomers with higher hydrophilicity and improved hydrolytic stability: II. Properties of N-acryloylaminopropanol. ELECTROPHORESIS. 1996;17:732–737. doi: 10.1002/elps.1150170419. [DOI] [PubMed] [Google Scholar]
- 26.Chen S, Li L, Zhao C, Zheng J. Surface hydration: Principles and applications toward low-fouling/nonfouling biomaterials. Polymer. 2010;51:5283–5293. [Google Scholar]
- 27.Gomez JE, Sandoval JE. The effect of conditioning of fused-silica capillaries on their electrophoretic performance. ELECTROPHORESIS. 2008;29:381–392. doi: 10.1002/elps.200700577. [DOI] [PubMed] [Google Scholar]
- 28.Gomez JE, Sandoval JE. New Approaches to Prepare Hydride Silica. Analytical Chemistry. 2010;82:7444–7451. doi: 10.1021/ac101608v. [DOI] [PubMed] [Google Scholar]
- 29.Gomez JE, Navarro FH, Sandoval JE. Novel 3-hydroxypropyl-bonded phase by direct hydrosilylation of allyl alcohol on amorphous hydride silica. ELECTROPHORESIS. 2014;35:2579–2586. doi: 10.1002/elps.201400216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Matyjaszewski K, et al. Polymers at Interfaces: Using Atom Transfer Radical Polymerization in the Controlled Growth of Homopolymers and Block Copolymers from Silicon Surfaces in the Absence of Untethered Sacrificial Initiator. Macromolecules. 1999;32:8716–8724. [Google Scholar]
- 31.Wang XS, Armes SP. Facile Atom Transfer Radical Polymerization of Methoxy-Capped Oligo(ethylene glycol) Methacrylate in Aqueous Media at Ambient Temperature. Macromolecules. 2000;33:6640–6647. [Google Scholar]
- 32.Ohno K, Morinaga T, Koh K, Tsujii Y, Fukuda T. Synthesis of Monodisperse Silica Particles Coated with Well-Defined, High-Density Polymer Brushes by Surface-Initiated Atom Transfer Radical Polymerization. Macromolecules. 2005;38:2137–2142. [Google Scholar]
- 33.Robinson KL, Khan MA, de Paz Banez MV, Wang XS, Armes SP. Controlled Polymerization of 2-Hydroxyethyl Methacrylate by ATRP at Ambient Temperature. Macromolecules. 2001;34:3155–3158. [Google Scholar]
- 34.Sandoval JE. Equation for calculating surface coverage from end-capping of chromatographic bonded phases. Journal of Chromatography A. 1999;852:375–381. doi: 10.1016/s0021-9673(99)00629-9. [DOI] [PubMed] [Google Scholar]
- 35.Williams BA, Vigh G. Fast, Accurate Mobility Determination Method for Capillary Electrophoresis. Analytical Chemistry. 1996;68:1174–1180. doi: 10.1021/ac950968r. [DOI] [PubMed] [Google Scholar]
- 36.Sandoval JE, Chen S-M. Method for the Accelerated Measurement of Electroosmosis in Chemically Modified Tubes for Capillary Electrophoresis. Analytical Chemistry. 1996;68:2771–2775. doi: 10.1021/ac9511509. [DOI] [PubMed] [Google Scholar]
- 37.Hempe JM, Granger JN, Craver RD. Capillary isoelectric focusing of hemoglobin variants in the pediatric clinical laboratory. ELECTROPHORESIS. 1997;18:1785–1795. doi: 10.1002/elps.1150181013. [DOI] [PubMed] [Google Scholar]
- 38.Hempe JM, Craver RD. Separation of hemoglobin variants with similar charge by capillary isoelectric focusing: Value of isoelectric point for identification of common and uncommon hemoglobin variants. ELECTROPHORESIS. 2000;21:743–748. doi: 10.1002/(SICI)1522-2683(20000301)21:4<743::AID-ELPS743>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 39.Kim J-B, Bruening ML, Baker GL. Surface-Initiated Atom Transfer Radical Polymerization on Gold at Ambient Temperature. Journal of the American Chemical Society. 2000;122:7616–7617. [Google Scholar]
- 40.Jones DM, Brown AA, Huck WTS. Surface-Initiated Polymerizations in Aqueous Media: Effect of Initiator Density. Langmuir. 2002;18:1265–1269. [Google Scholar]
- 41.Ma H, et al. Surface Initiated Polymerization from Substrates of Low Initiator Density and Its Applications in Biosensors. ACS Applied Materials & Interfaces. 2010;2:3223–3230. doi: 10.1021/am1006832. [DOI] [PubMed] [Google Scholar]
- 42.Espinal JH, Gomez JE, Sandoval JE. Closer look at the operating definition of protein recovery in CE. ELECTROPHORESIS. 2013;34:1141–1147. doi: 10.1002/elps.201200514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen H, et al. Synthesis and Characterization of Antifouling Poly(Nacryloylaminoethoxyethanol) with Ultralow Protein Adsorption and Cell Attachment. Langmuir. 2014;30:10398–10409. doi: 10.1021/la502136q. [DOI] [PubMed] [Google Scholar]
- 44.Schlenoff JB. Zwitteration: Coating Surfaces with Zwitterionic Functionality to Reduce Nonspecific Adsorption. Langmuir. 2014;30:9625–9636. doi: 10.1021/la500057j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.