Fig. 6.
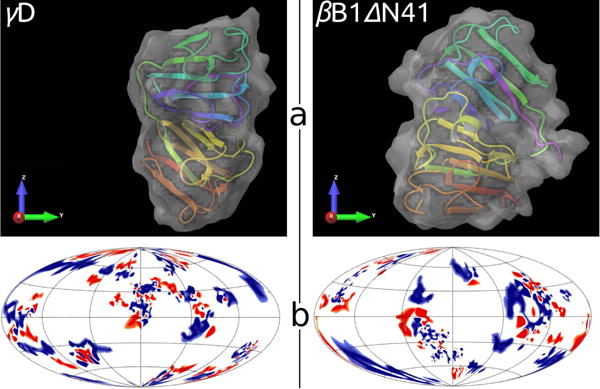
Comparison between γD (left) and βB1ΔN41 (right) crystallins. Panel a: tertiary structures embedded into solvent accessible area, generated by Schrödinger software63. Panel b: charged amino acid residues on surfaces of proteins; positive – blue, negative – red, neutral – white. The location of residues is shown in the Hammer projection64, having the polar and the azimuthal angles as the principle coordinate axes. An implementation of this projection was proposed by Koromyslova65 et al and modified by us, as described in SI.
