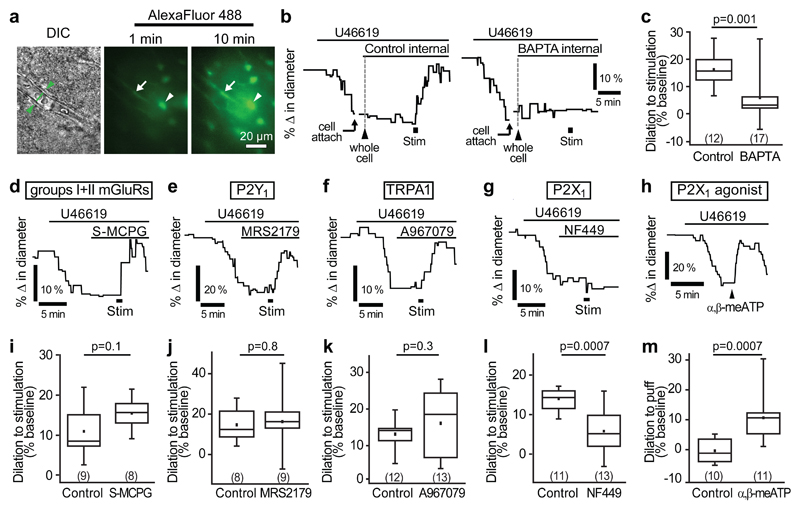
Figure 2. P2X1-evoked astrocyte Ca2+ signalling mediates capillary-level neurovascular coupling.
(a) A DIC image of a cortical capillary (left panel) and the AlexaFluor 488 fill of the astrocyte network after whole-cell patch-clamp dialysis for 1 min (middle) and 10 mins (right) after breaking into the cell. Whole-cell patch-clamped cell is indicated by arrowhead and endfoot indicated by arrow. (b) Example traces and (c) mean data demonstrating that stimulation-evoked capillary dilation is intact when the astrocyte network is dialyzed with a control internal solution containing 1 mM EGTA but significantly reduced when filled with 30 mM BAPTA, a fast Ca2+-chelator. (d-f) An inhibitor of group I and II mGluRs, S-MCPG (1 mM; d), the P2Y1 blocker MRS2179 (25 μM; e) and the TRPA1 blocker A967079 (10 μM; f) do not block stimulation-evoked capillary dilation. (g) The P2X1 blocker NF449 (100 nM) significantly reduced stimulation-evoked capillary dilation. (h) Puff-application of the P2X1 agonist α,β-methylene ATP (α,β-meATP, 100 µM) to the neuropil downstream of the vessel induces capillary dilation. (i-l) Quantification of the effect of S-MCPG (i), MRS2179 (j), A967079 (k) and NF449 (l) on capillary dilation. (m) Mean response of capillaries in experiments like those in h, puffing external solution (control) or α,β-methylene ATP. Change in diameter in control experiments was measured as a 30 s average centred around the largest response seen between 30 and 120 s after puff of α,β-methylene ATP. Data are shown as box and whisker plots as defined in the Statistics part of the Methods.