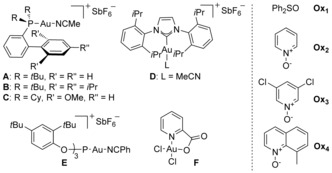
Table 1.
Gold(I)‐catalyzed oxidative reaction of 6 b to give 1‐phenylbarbaralone (1 b).
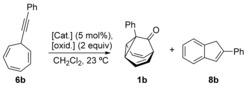
| Entry | [Cat.] | Oxid. | Time [h] | 1 b Yield [%][a] | 8 b Yield [%][a] |
|---|---|---|---|---|---|
| 1 | A | Ox1 | 2.5 | 12 | 58 |
| 2 | A | Ox2 | 16 | 5 | – |
| 3 | A | Ox3 | 2.5 | 50 (50)[b] | 28 |
| 4 | A | Ox4 | 16 | 23 | – |
| 5 | B | Ox3 | 3 | 30 | 36 |
| 6 | C | Ox3 | 3 | 32 | 42 |
| 7 | D | Ox1 | 3 | (83)[b] | – |
| 8 | D | Ox2 | 24 | 2 | – |
| 9 | D | Ox3 | 3 | 64 | – |
| 10 | D | Ox4 | 3 | 7 | – |
| 11 | E | Ox1 | 2.5 | 20 | – |
| 12 | E | Ox3 | 2.5 | 30 | – |
| 13 | F | Ox1 | 5 | 14 | 61 |
| 14 | F | Ox3 | 5 | 61 | – |
| 15 | MeSO3H[c] | Ox3 | 2.5 | complex mixture | |
| 16 | Zn(OTf)2 [d] | Ox3 | 24 | starting material | |
[a] Yields determined by 1H NMR using mesitylene as an internal standard. [b] Yield of isolated products. [c] 4 equiv [d] 10 mol %. Catalyst (cat.), oxidant (oxid.).