Figure 2.
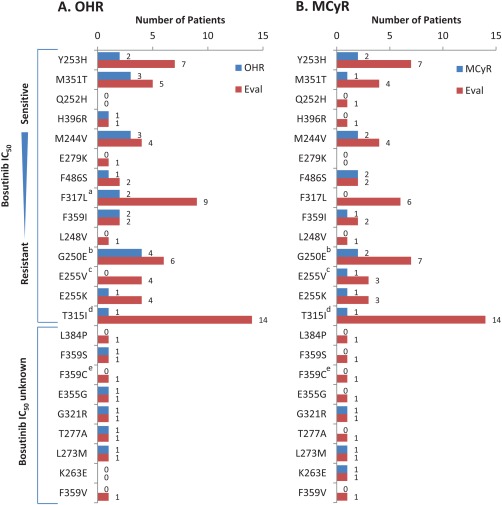
OHR (A) and MCyR (B) by individual baseline Bcr‐Abl mutations in AP, BP, and ALL cohorts. Individual patients may have had more than one detected mutation. Bosutinib IC50 concentrations were based on data from 34. aIncludes one evaluable ALL patient with a F317L mutation who did not achieve a response; bincludes one evaluable ALL patient with a G250E mutation who did not achieve a response; cincludes one evaluable ALL patient with a E255V mutation who did not achieve a response; dincludes three evaluable ALL patients with a T315I mutation who did not achieve a response; eincludes one evaluable ALL patient with a F359C mutation who did not achieve a response. Eval, number of patients with each baseline mutation who had a valid baseline efficacy assessment for the respective endpoint; IC50, half‐maximal inhibitory concentration; MCyR, major cytogenetic response; OHR, overall hematologic response.
