Figure 3.
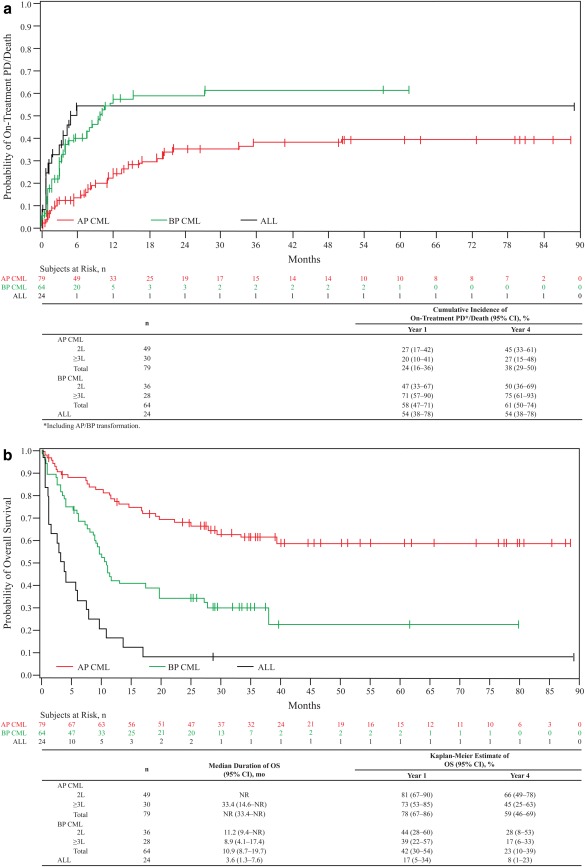
Cumulative incidence of PD/death adjusting for the competing risk of treatment discontinuation without PD/death (A) and overall survival (B). Criteria for PD included transformation to AP/BP CML, increasing white blood cell count (doubling over ≥1 month with second count >20 × 109/L and confirmed ≥1 week later, or loss of confirmed CHR or unconfirmed MCyR. OS was calculated from the date of first study dosing to the date of death, with patients without events censored at the last contact, and was evaluated throughout the 2‐year follow‐up period after treatment discontinuation. The median follow‐up was 28.4 (0.3–88.6), 10.4 (0.4–79.9), and 3.6 (0.4–89.2) months for AP, BP, and ALL patients, respectively. 2L, Second‐line (prior imatinib only); ≥3L, third‐/fourth‐line (imatinib followed by dasatinib and/or nilotinib); ALL, acute lymphoblastic leukemia; AP, accelerated phase; BP, blast phase; CI, confidence interval; CML, chronic myeloid leukemia; NR, not reached; OS, overall survival; Ph+, Philadelphia chromosome‐positive; PD, progressive disease.
