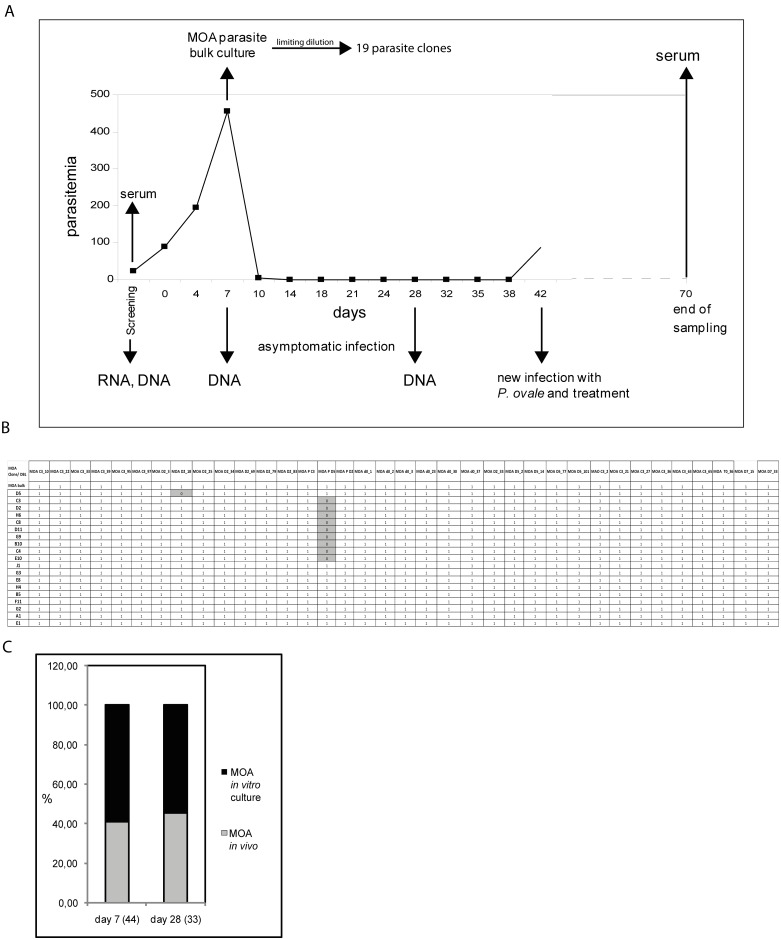
Fig 1. In vitro and in vivo analysis of a chronic P. falciparum infection in a semi-immune individual.
(A) Parasitemia curve of the asymptomatic chronic P. falciparum infection of an adult semi-immune Gabonese individual over 70 days and timeline of the in vivo investigation. The patient was asymptomatic from day 0 to day 42, at which point a new infection with P. ovale was diagnosed and treated according to local guidelines. DNA, RNA, cryopreserved parasites and sera employed in this analysis are indicated by arrows. Blood parasitemia is quantified on the y-axis. P. falciparum parasites were detected from day 0 to day 10 by light microscopy. From day 7 to day 38 sub microscopic parasitemia was documented by PCR twice per week. For in vitro analysis parasites from day 7 were brought into tissue culture (MOA bulk) and 19 in vitro clones were generated by limiting dilution in two independent cloning experiments. (B) Matrix of PCR results with 36 DBL specific primers on DNA of in vitro MOA bulk culture and 19 MOA clones. 1 indicates amplification of the target sequence, 0 indicates absence of a reaction product. All 36 DBLs were amplified from MOA bulk and the clones MOA J1, G3, E8, H4, B5, F11, G2, A1 and E1. In the remaining clones 35 DBLs were successfully amplified. In the clone MOA D5 the DBL MOA D2_18 could not be amplified. In the clones MOA C3, D2, H6, C8, D11, G9, B10, C4, E10 the DBL MOA P D5 could not be amplified (grey). (C) Results of DBL shotgun cloning of DNA from patient blood of day 7 and day 28. Numbers in brackets shown below the x-axis refer to the total number of var DBL sequences identified on these days. Sequences that were identified exclusively in DNA from patient blood are designated as "in vivo" MOA (grey). The proportion of MOA “in vitro” DBLs is represented by the black part of the bars. MOA in vitro var DBLs were detected at the same proportion (59% (26 of 44)) on day 7 and day 28 (55% (18 of 33)).