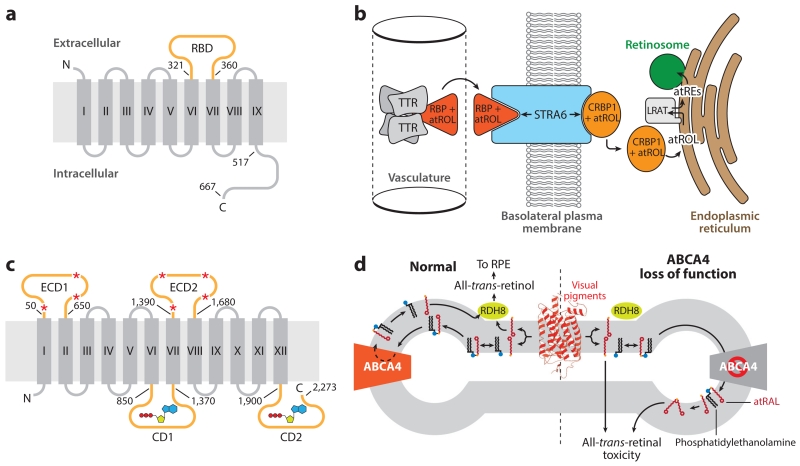
Figure 5.
Structure and function of retinoid transporters essential for retinal health and function. (a) Topology diagram of the holo-RBP receptor, STRA6. The RBD (orange) resides between transmembrane helices VI and VII. Numbering indicates positions within the human amino acid sequence. (b) The role of STRA6 in the uptake of atROL from holo-RBP into a target tissue, such as the RPE. (c) Topological diagram of ABCA4. The large ECDs are glycosylated at multiple positions, as indicated by red asterisks. Two CDs contain Walker A and B motifs responsible for adenosine triphosphate binding and hydrolysis (shown in a schematic representation) that drive the transport function of the protein. Numbering indicates positions within the human amino acid sequence. (d) Role of ABCA4 in the metabolism of atRAL. atRAL (red sticks) released from photoactivated visual pigments (red schematic) into the exocytoplasmic leaflet of the disk membrane is inaccessible to RDH8, the enzyme that converts atRAL into atROL. atRAL readily forms a Schiff base conjugate with phosphatidylethanolamine (black sticks with blue headgroup). The Ret-PE adduct is translocated to the cytosolic leaflet through the action of ABCA4, where it dissociates to yield free atRAL that can be metabolized by RDH8 to nonelectrophilic atROL. Loss-of-function mutations in ABCA4, causative of Stargardt disease, result in the accumulation of atRAL and Ret-PE, which can undergo secondary reactions to form potentially toxic bis-retinoids and protein–retinoid conjugates that ultimately lead to retinal degeneration. Panels b and d adapted from Kiser et al. (2014). Abbreviations: ABCA4, adenosine triphosphate-binding cassette transporter 4; atRAL, all-trans-retinal; atRE, all-trans-retinol ester(s); atROL, all-trans-retinol; CD, cytosolic domain; CRBP, cellular retinal-binding protein; ECD, extracellular domain; LRAT, lecithin:retinol acyltransferase; RDH8, retinol dehydrogenase 8; RBP, retinol-binding protein; RBD, RBP-binding domain; Ret-PE, retinal-phosphatidylethanolamine Schiff base adduct; RPE, retinal pigment epithelium; STRA6, stimulated by retinoic acid 6; TTR, transthyretin.