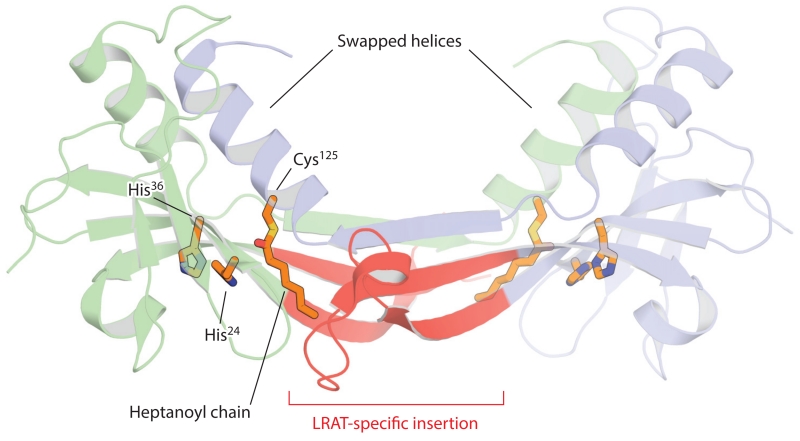
Figure 7.
Crystal structure of the lecithin:retinol acyltransferase (LRAT)–H-Ras-like tumor suppressor 3 chimera. The two chains of the homodimer are shown in a schematic representation (blue and green). The LRAT-specific insertion (red) forms a β-sheet that mediates dimer formation. This region also provides a pathway for the exchange of the C-terminal α-helical regions within the dimer pair. The histidine (His) and cysteine (Cys) residues constituting the catalytic triad are shown as sticks. The catalytic Cys125 residue is acylated as a result of the crystals being grown in the presence of the acyl donor, diheptanoyl phosphatidylcholine. The figure was generated with PyMol (Schrödinger, https://www.pymol.org/).