Figure 8.
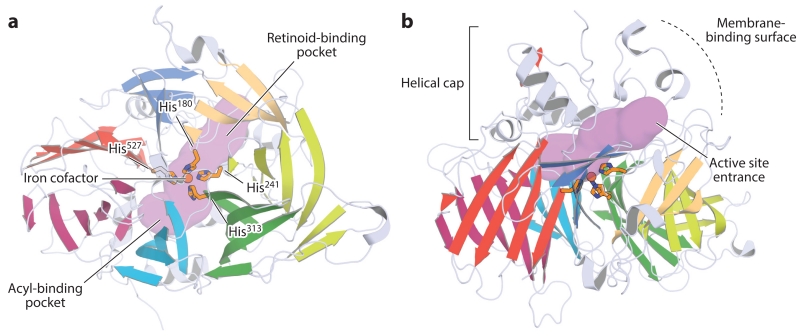
Orthogonal views of the crystal structure of retinal pigment epithelium–specific 65 kDa protein. (a) View down the β-propeller axis, with the seven blades indicated by distinct colors. The iron cofactor (orange sphere) is coordinated on the propeller axis by four conserved histidine (His) residues. The purple surface delineates the active site pocket. (b) Rotating the structure by 90° along the horizontal axis shows the helical cap on the top face of the β-propeller. The active site entrance is surrounded by a patch of mostly hydrophobic residues that mediate the membrane binding critical for substrate uptake. The structural representations were generated with PyMol (Schrödinger, https://www.pymol.org/) using the coordinates deposited under Protein Data Bank accession code 4F2Z.