Abstract
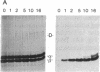
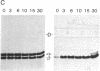
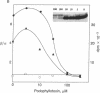
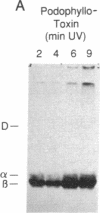
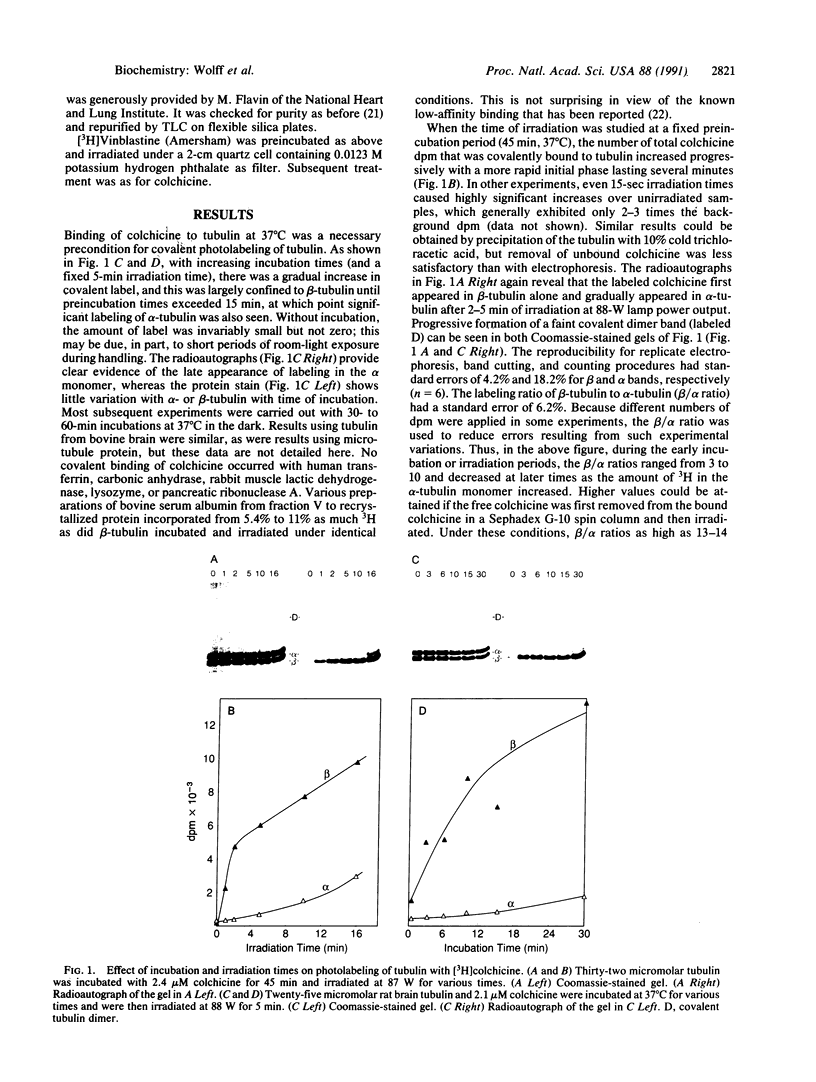
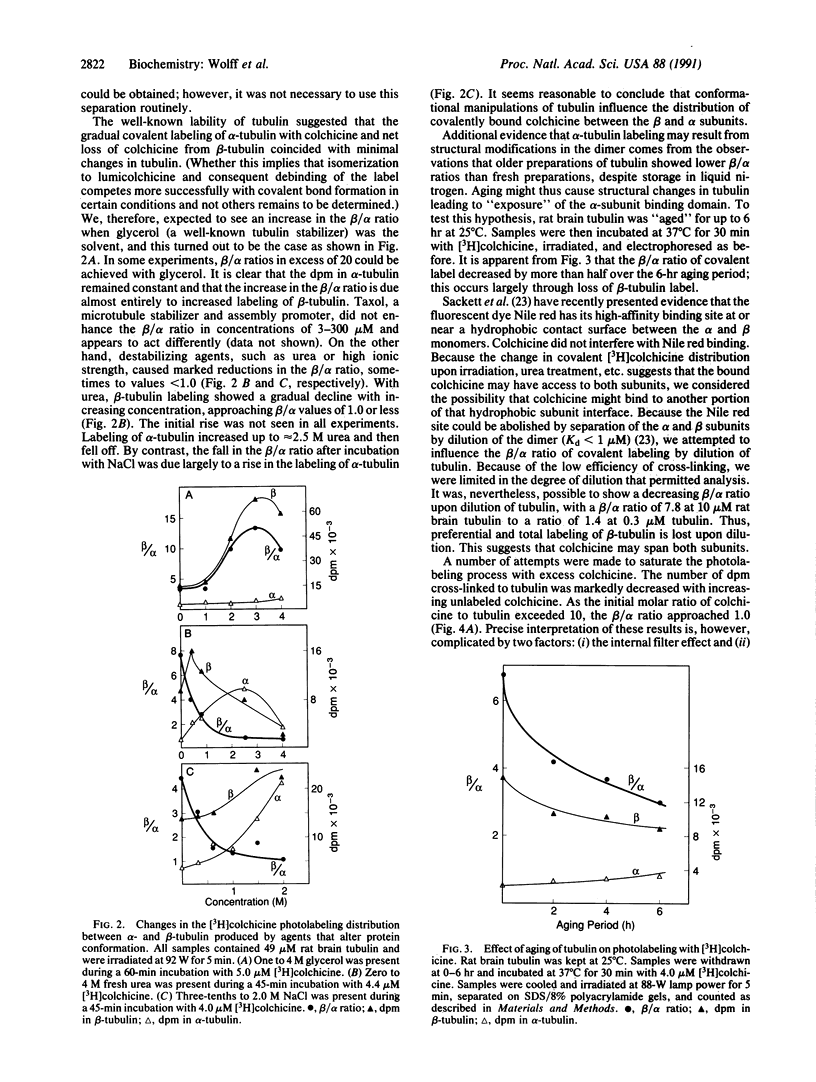
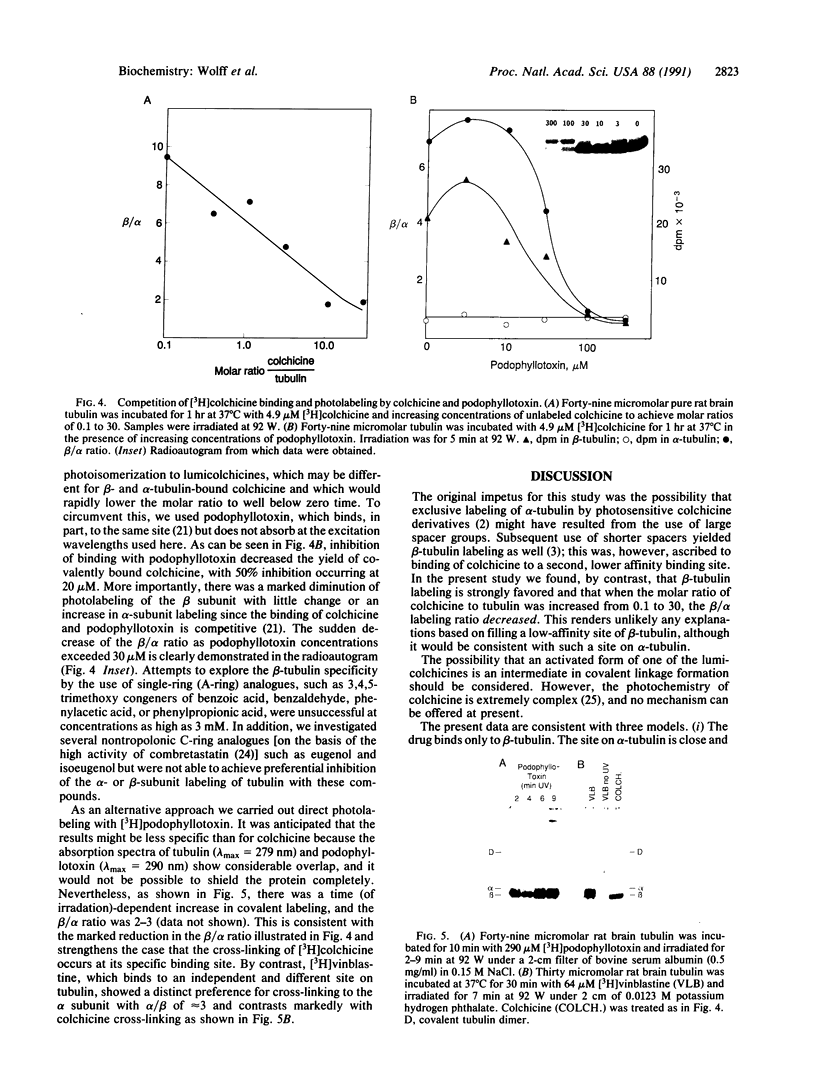
Ultraviolet irradiation of the [3H]colchicine-tubulin complex leads to direct photolabeling of tubulin with low but practicable efficiency. The bulk (70% to greater than 90%) of the labeling occurs on beta-tubulin and appears early after irradiation, whereas alpha-tubulin is labeled later. The labeling ratio of beta-tubulin to alpha-tubulin (beta/alpha ratio) is reduced by prolonged incubation, prolonged irradiation, urea, high ionic strength, the use of aged tubulin, dilution of tubulin, or large concentrations of colchicine or podophyllotoxin. Glycerol increases the beta/alpha ratio. Limited data with [3H]podophyllotoxin show that it covalently bound with a similar beta/alpha distribution. Vinblastine, on the other hand, exhibits preferential attachment to alpha-tubulin. The possibilities that colchicine binds at the interface between alpha-tubulin and beta-tubulin, that the drug spans this interface, and that both subunits may contribute to the binding site are suggested.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amrhein N., Filner P. Sensitization of colchicine binding protein to ultraviolet light by bound colchicine. FEBS Lett. 1973 Jun 15;33(1):139–142. doi: 10.1016/0014-5793(73)80178-4. [DOI] [PubMed] [Google Scholar]
- Borisy G. G., Olmsted J. B., Klugman R. A. In vitro aggregation of cytoplasmic microtubule subunits. Proc Natl Acad Sci U S A. 1972 Oct;69(10):2890–2894. doi: 10.1073/pnas.69.10.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabral F., Sobel M. E., Gottesman M. M. CHO mutants resistant to colchicine, colcemid or griseofulvin have an altered beta-tubulin. Cell. 1980 May;20(1):29–36. doi: 10.1016/0092-8674(80)90231-7. [DOI] [PubMed] [Google Scholar]
- Cortese F., Bhattacharyya B., Wolff J. Podophyllotoxin as a probe for the colchicine binding site of tubulin. J Biol Chem. 1977 Feb 25;252(4):1134–1140. [PubMed] [Google Scholar]
- Detrich H. W., 3rd, Williams R. C., Jr, Wilson L. Effect of colchicine binding on the reversible dissociation of the tubulin dimer. Biochemistry. 1982 May 11;21(10):2392–2400. doi: 10.1021/bi00539a018. [DOI] [PubMed] [Google Scholar]
- Donigian D. W., Owellen R. J. Interaction of vinblastine, vincristine and colchicine with serum proteins. Biochem Pharmacol. 1973 Sep 1;22(17):2113–2119. doi: 10.1016/0006-2952(73)90110-x. [DOI] [PubMed] [Google Scholar]
- Floyd L. J., Barnes L. D., Williams R. F. Photoaffinity labeling of tubulin with (2-nitro-4-azidophenyl)deacetylcolchicine: direct evidence for two colchicine binding sites. Biochemistry. 1989 Oct 17;28(21):8515–8525. doi: 10.1021/bi00447a037. [DOI] [PubMed] [Google Scholar]
- Hamel E., Lin C. M. Separation of active tubulin and microtubule-associated proteins by ultracentrifugation and isolation of a component causing the formation of microtubule bundles. Biochemistry. 1984 Aug 28;23(18):4173–4184. doi: 10.1021/bi00313a026. [DOI] [PubMed] [Google Scholar]
- Ide G., Engelborghs Y. Fluorescence quenching and induced dissociation of the tubulin-colchicine complex by iodide. J Biol Chem. 1981 Nov 25;256(22):11684–11687. [PubMed] [Google Scholar]
- Keates R. A., Sarangi F., Ling V. Structural and functional alterations in microtubule protein from Chinese hamster ovary cell mutants. Proc Natl Acad Sci U S A. 1981 Sep;78(9):5638–5642. doi: 10.1073/pnas.78.9.5638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeir A., Engelborghs Y. A fluorescence stopped flow study of colchicine binding to tubulin. J Biol Chem. 1981 Apr 10;256(7):3279–3282. [PubMed] [Google Scholar]
- Lin C. M., Ho H. H., Pettit G. R., Hamel E. Antimitotic natural products combretastatin A-4 and combretastatin A-2: studies on the mechanism of their inhibition of the binding of colchicine to tubulin. Biochemistry. 1989 Aug 22;28(17):6984–6991. doi: 10.1021/bi00443a031. [DOI] [PubMed] [Google Scholar]
- Merrill B. M., Williams K. R., Chase J. W., Konigsberg W. H. Photochemical cross-linking of the Escherichia coli single-stranded DNA-binding protein to oligodeoxynucleotides. Identification of phenylalanine 60 as the site of cross-linking. J Biol Chem. 1984 Sep 10;259(17):10850–10856. [PubMed] [Google Scholar]
- Pedersen S. E., Cohen J. B. d-Tubocurarine binding sites are located at alpha-gamma and alpha-delta subunit interfaces of the nicotinic acetylcholine receptor. Proc Natl Acad Sci U S A. 1990 Apr;87(7):2785–2789. doi: 10.1073/pnas.87.7.2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roach M. C., Bane S., Ludueña R. F. The effects of colchicine analogues on the reaction of tubulin with iodo[14C]acetamide and N,N'-ethylenebis(iodoacetamide). J Biol Chem. 1985 Mar 10;260(5):3015–3023. [PubMed] [Google Scholar]
- Rousset B., Bernier-Valentin F., Wolff J., Roux B. Alterations in tubulin immunoreactivity; relation to secondary structure. Eur J Biochem. 1983 Mar 1;131(1):31–39. doi: 10.1111/j.1432-1033.1983.tb07228.x. [DOI] [PubMed] [Google Scholar]
- Sackett D. L., Knutson J. R., Wolff J. Hydrophobic surfaces of tubulin probed by time-resolved and steady-state fluorescence of nile red. J Biol Chem. 1990 Sep 5;265(25):14899–14906. [PubMed] [Google Scholar]
- Sackett D. L., Zimmerman D. A., Wolff J. Tubulin dimer dissociation and proteolytic accessibility. Biochemistry. 1989 Mar 21;28(6):2662–2667. doi: 10.1021/bi00432a045. [DOI] [PubMed] [Google Scholar]
- Schmitt H., Atlas D. Specific affinity labelling of tubulin with bromocolchicine. J Mol Biol. 1976 Apr 25;102(4):743–758. doi: 10.1016/0022-2836(76)90289-8. [DOI] [PubMed] [Google Scholar]
- Serrano L., Avila J., Maccioni R. B. Limited proteolysis of tubulin and the localization of the binding site for colchicine. J Biol Chem. 1984 May 25;259(10):6607–6611. [PubMed] [Google Scholar]
- Sheer D. G., Cahnmann H. J., Nikodem V. M. Molecular size of the nuclear thyroid hormone receptor. Biochim Biophys Acta. 1987 Aug 19;930(1):101–106. doi: 10.1016/0167-4889(87)90161-3. [DOI] [PubMed] [Google Scholar]
- Sheir-Neiss G., Lai M. H., Morris N. R. Identification of a gene for beta-tubulin in Aspergillus nidulans. Cell. 1978 Oct;15(2):639–647. doi: 10.1016/0092-8674(78)90032-6. [DOI] [PubMed] [Google Scholar]
- Sloboda R. D., Rosenbaum J. L. Purification and assay of microtubule-associated proteins (MAPs). Methods Enzymol. 1982;85(Pt B):409–416. doi: 10.1016/0076-6879(82)85041-6. [DOI] [PubMed] [Google Scholar]
- Williams R. F., Mumford C. L., Williams G. A., Floyd L. J., Aivaliotis M. J., Martinez R. A., Robinson A. K., Barnes L. D. A photoaffinity derivative of colchicine: 6'-(4'-azido-2'-nitrophenylamino)hexanoyldeacetylcolchicine. Photolabeling and location of the colchicine-binding site on the alpha-subunit of tubulin. J Biol Chem. 1985 Nov 5;260(25):13794–13802. [PubMed] [Google Scholar]
- Wilson L., Friedkin M. The biochemical events of mitosis. I. Synthesis and properties of colchicine labeled with tritium in its acetyl moiety. Biochemistry. 1966 Jul;5(7):2463–2468. doi: 10.1021/bi00871a042. [DOI] [PubMed] [Google Scholar]