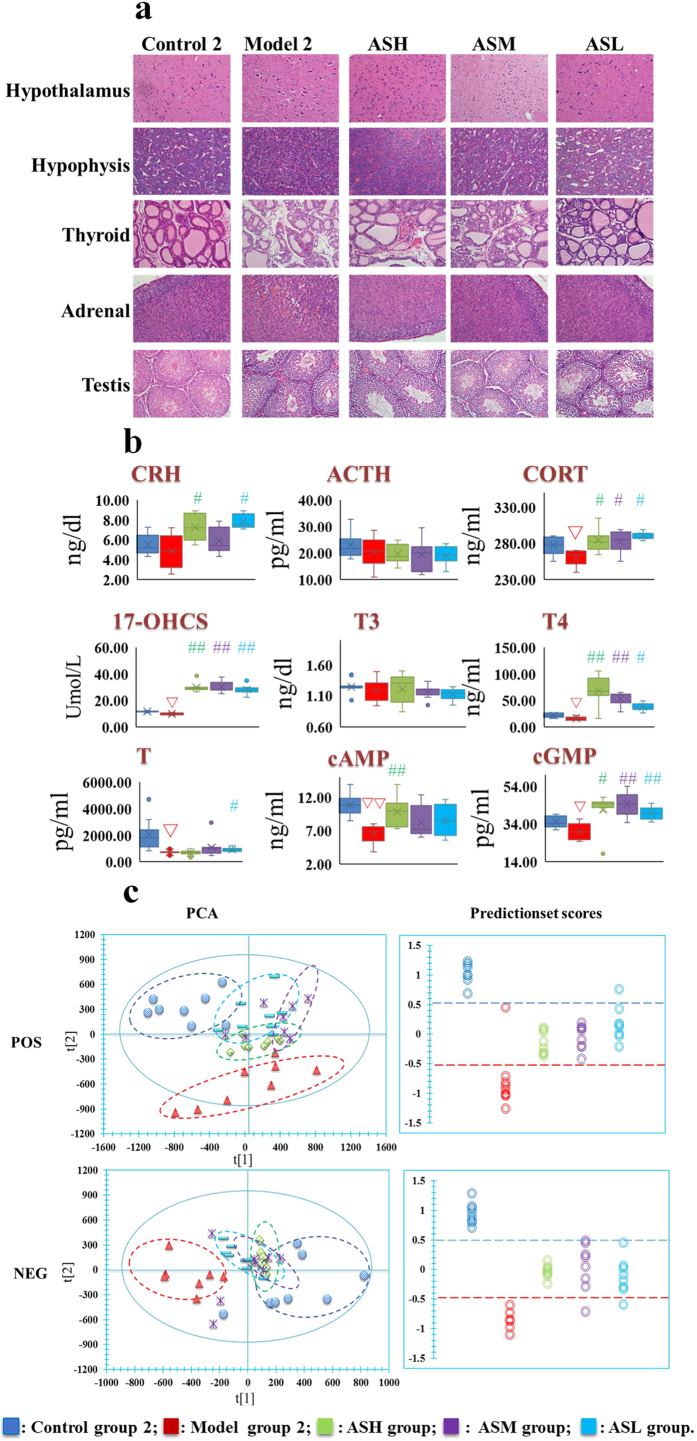
Figure 2. The evaluation system diagrams of the therapeutic effects of AS1350 on Kidney-Yang Deficiency Syndrome (day 43).
(a) H&E staining results of hypothalamus, pituitary, thyroid, adrenal glands, and testes in the rats of control group 2, model group 2, and AS1350-treated group as observed by visible light microscope: all of the evidence showed that the extent of the treatment effect of AS1350 was proved by morphology results: neuronal cells in hypothalamus and basophils were increased, adrenocortical cells were arranged evenly and the atrophic cells were decreased, thyroid follicles were large and plump as well as the interstitial hyperplasia also being reduced to a regular arrangement. (b) The biochemical characteristics used for evaluation of the therapeutic effects of AS1350 on Kidney-Yang Deficiency Syndrome. Box and whisker diagrams comparing the level of hormones in neuroendocrine systems with each group (control group 1, model group 2, and AS1350-treated groups). After the treatment of AS1350, various levels of CORT, 17-OHCS, T, T4, cAMP, and cGMP in treatment groups returned to the level of control group 2 (1-way ANOVA with a Bonferroni correction; ∇ significant difference from control group 2 at p < 0.05/4, ∇∇ significant difference from control group 2 at p < 0.01/4; #significant difference from model group 2 at p < 0.05/4, ## significant difference from model group 2 at p < 0.01/4.). (c) Metabolomic in vivo evaluation of AS1350. PCA plots showed separation between control group 2, model group 2, and AS1350-treated. AS1350-treated groups were closer to control group 2 than model group 2, which suggested that AS1350 could reverse the pathological process of KYDS. The prediction set also annotated the therapeutic effect of each dose of AS1350, which showed the same results as the PCA plots.