Abstract
The diverse anticancer utility of cisplatin has stimulated significant interest in the development of additional platinum-based therapies, resulting in several analogues receiving clinical approval worldwide. However, due to structural and mechanistic similarities, the effectiveness of platinum-based therapies is countered by severe side-effects, narrow spectrum of activity and the development of resistance. Nonetheless, metal complexes offer unique characteristics and exceptional versatility, with the ability to alter their pharmacology through facile modifications of geometry and coordination number. This has prompted the search for metal-based complexes with distinctly different structural motifs and non-covalent modes of binding with a primary aim of circumventing current clinical limitations. This review discusses recent advances in platinum and other transition metal-based complexes with mechanisms of action involving intercalation. This mode of DNA binding is distinct from cisplatin and its derivatives. The metals focused on in this review include Pt, Ru and Cu along with examples of Au, Ni, Zn and Fe complexes; these complexes are capable of DNA intercalation and are highly biologically active.
Keywords: cancer, intercalate, transition metals, DNA, cytotoxicity, DNA binding, platinum
1. Introduction
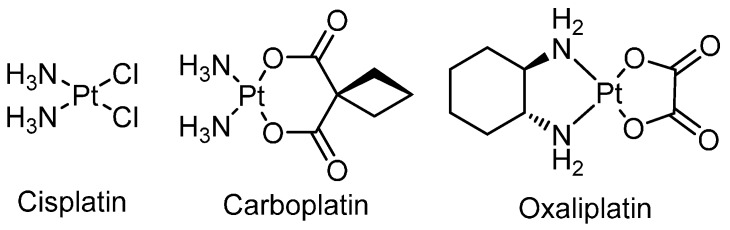
The anticancer activity of the platinum-based complex, cisplatin (Figure 1), was discovered in the 1960s and has since been used extensively for the treatment of various cancers including ovarian, testicular, lung and breast cancer [1,2,3]. This activity, however, is moderated by dose-limiting side-effects (nephro-, neuro- and ototoxicity) and development of resistance (acquired or intrinsic) [4]. In attempts to overcome the aforementioned, thousands of analogues have been synthesised, however, of these, only carboplatin (Figure 1) and oxaliplatin (Figure 1) have been approved for worldwide use. These complexes exhibit different side-effects and overcome some cisplatin resistance, respectively, although otherwise they demonstrate no significant improvements in efficacy overall [5,6]. This may be attributed to their similar geometrical configurations as they conform to the original structure-activity relationships that were reported requirements for exhibiting anticancer activity i.e., a neutral platinum(II) complex containing am(m)ine ligands and leaving group(s) that can be replaced during aquation [7,8]. The anticancer activity of cisplatin is generally attributed to its coordinative interaction with DNA. Upon entering the cell, the chloride ligands are substituted by water, forming strong electrophiles that can readily interact with nucleophilic bases of nucleic acids [6]. This results in the formation of covalently bound monofunctional and bifunctional adducts (mainly 1,2-intrastrand cross-links) that induce a conformational change to DNA which, through a series of events, ultimately leads to apoptosis (Figure 2) [6].
Figure 1.
Chemical structures of cisplatin, carboplatin and oxaliplatin.
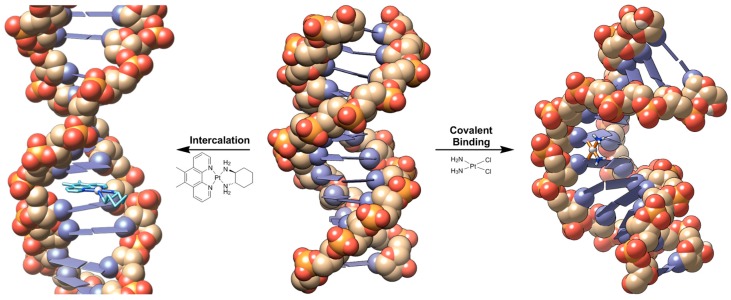
Figure 2.
Schematic representation of a metal complex interacting with DNA, resulting in elongation of the double-helix (left, sourced from Protein Data Bank (PDB) file 2MG8 [9] with metal complex inserted manually) and cisplatin covalently binding to DNA, causing the double helix to bend (right, sourced from PDB 1AIO) [10]. Central DNA figure sourced from PDB file 1D86 [11]. Oxygen is orange, phosphorous is yellow, carbon is cream/white and nitrogen is blue/purple. The base pairs are also represented as blue/purple rectangular panels.
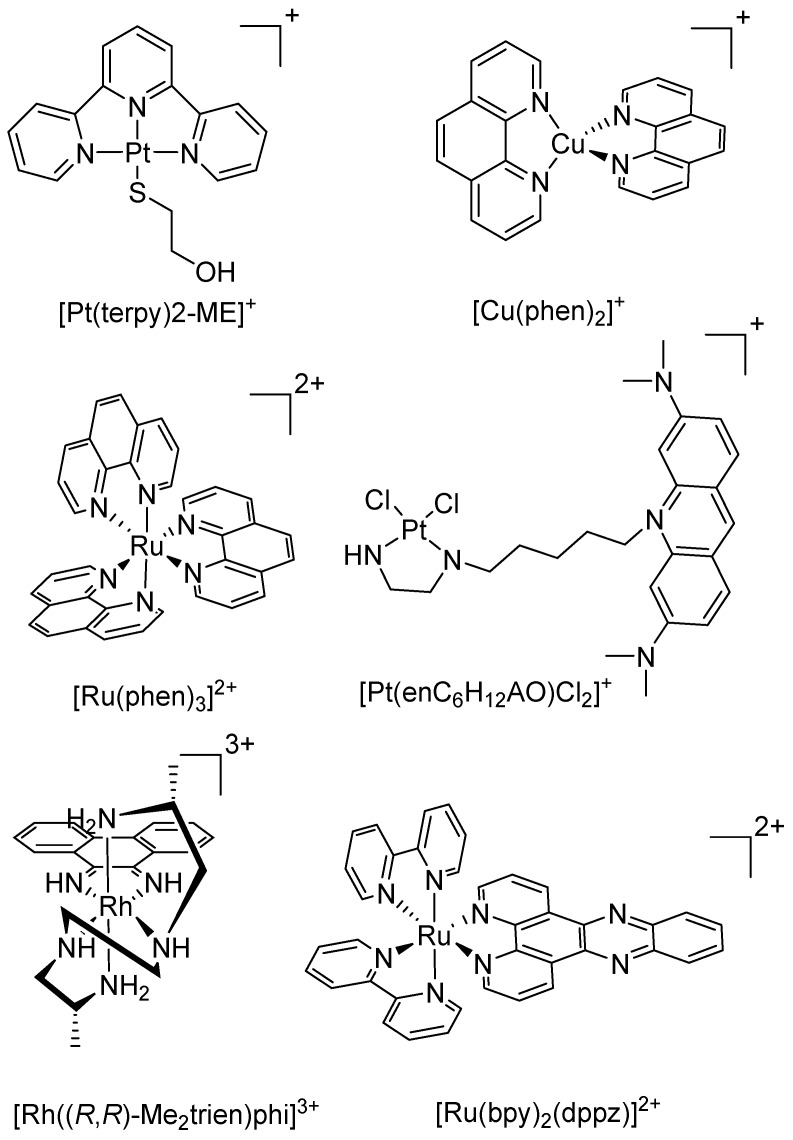
With the aim of developing metal-based complexes that exhibit improved pharmacological properties, efforts have been made to develop complexes with different modes of action and higher efficacy relative to cisplatin derivatives, including complexes that target cellular components other than DNA, complexes combined with delivery or targeting agents, or complexes that interact with DNA through non-covalent methods. A promising series of atypical anticancer metal complex are metallointercalators. Intercalation is the insertion of a complex within two adjacent base pairs of DNA [12]. Intercalators generally incorporate electron deficient, planar aromatic rings where non-covalent interactions with DNA are facilitated and stabilised through π–π stacking and dipole-dipole interactions, causing DNA to unwind and extend in order to accommodate the metal complex between the base pairs (Figure 2) [13,14]. This has the potential to circumvent recognition of repair mechanisms that lead to the resistance seen with cisplatin and its analogues. Transition metals deliver utility in anticancer drug design as they exhibit widely diverse geometries, coordination numbers, and selection of ligands that will coordinate, all with subtly different redox potentials and stabilities. Transition metal intercalators have been in development for decades; the original platinum complex [Pt(terpy)(2-ME)]+ (where 2-ME = 2-mercaptoethanol) was shown to bind strongly to DNA via intercalation, while a subsequent compound [Pt(enC6H12AO)Cl2]+ (where enC6H12AO is ethylenediamine tethered to acridine orange via an alkyl chain) bound with enhanced sequence-specificity for certain DNA sequences (Figure 3) [15,16]. The tetrahedral [Cu(phen)2]+ (where phen = 1,10-phenanthroline) was reported to be a potent inhibitor of polymerase I in Escherichia coli, which was achieved through DNA cleavage (Figure 3) [17]. The octahedral complex [Ru(phen)3]2+ is able to intercalate and unwind DNA as effectively as ethidium bromide while [Ru(bpy)2(dppz)]2+ (where bpy = 2,2’-bipyridine and dppz = dipyrido[3,2-a:2’,3’-c]phenazine) demonstrated enhanced luminescence upon intercalation with DNA, with the potential for use as a luminescent DNA probe (Figure 3) [18,19]. The rhodium complex, Δ-α-[Rh((R,R)-Me2trien)phi]3+ (where Me2trien = 2R,9R-diamino-4,7-diazadecane and phi = 9,10-phenanthrenequinone diamine), intercalates specifically to the major groove of the four base pair sequence 5′-TGCA-3′ and can also be photoactivated resulting in photoinduced oxidation of DNA (Figure 3) [20].
Figure 3.
Chemical structures of early transition metal intercalators.
Due to their intrinsic properties, the impact of transition metals on the binding properties of the intercalating ligand can be staggering [21,22,23]; for example, a nickel complex of porphyrin was found to bind to DNA by intercalation, however the zinc complex of the same porphyrin could only bind through surface interactions due to the presence of an extra axial aqua ligand [24]. This review covers recent advances in anticancer intercalating complexes of a variety of transition metals. In particular, we focus on complexes that have had recent developments within the past three years, are confirmed to intercalate with DNA, and also exhibit high cytotoxcicty toward cancerous cells.
2. Platinum
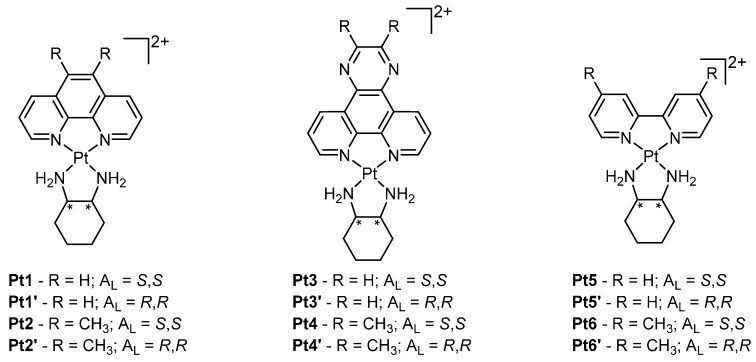
To date, the majority of platinum anticancer research has focused on the design of cisplatin analogues that covalently bind DNA. In contrast intercalators have received less attention; however, there are several recent examples of platinum intercalators that exhibit exceptionally high anticancer activity. A prominent series of complexes are composed of a general scaffold of [Pt(HL)(AL)]2+, where HL is a heterocyclic intercalating ligand and AL is a bidentate ancillary ligand [25,26]. These complexes include dipyrido[3,2-f:2’,3’-h]quinoxaline (dpq), 2,3-dimethyl-dpq (23Me2dpq), phen, 5,6-dimethyl-phen (56Me2phen), bpy or 4,4’-dimethyl-bpy (44Me2bpy) as the HL and either the S,S or R,R isomer of 1,2-diaminocyclohexane (S,S- or R,R-dach) as the AL (Figure 4).
Figure 4.
General structures of phen, dpq, bpy platinum intercalators. * indicates a stereocentre of the AL, either S or R. Counter ions have been omitted for clarity.
Interactions of these platinum complexes (PCs) with DNA have been studied using various spectroscopic techniques as well as mass spectrometry and isothermal titration calorimetry, which provided evidence for a GC-selective intercalative binding mode and DNA affinity in the range of ~104–106 M−1 [27]. In vitro cytotoxicity assays showed greater activity than cisplatin and its analogues against a range of cell lines with a number of complexes demonstrating low-nanomolar activity (Table 1) [27]. For complexes consisting of bpy (i.e., Pt5), phen (i.e., Pt1) or their derivatives thereof, a correlation was apparent between DNA binding affinity and cytotoxicity where a higher DNA binding affinity was directly proportional to increased cytotoxicity, indicating DNA binding influences the apoptotic activity of these PCs. However, DNA affinity is not the only factor governing the activity of these complexes as the choice of AL has a large effect. For example, complexes of S,S-dach (i.e., Pt1 and Pt3) displayed higher cytotoxicity than those of R,R-dach (i.e., Pt1’ and Pt3’), despite exhibiting the same DNA affinity [25].
Table 1.
In vitro cytotoxicity of Pt1–6 and Pt1’–6’ against L1210 (murine leukaemia) and Du145 (prostate cancer) and A2780 (human ovarian cancer) cell lines. IC50 is the concentration at which cell growth is inhibited by 50% over 72 h. Values taken from reference [27].
| Complex | IC50 (µM) | ||
|---|---|---|---|
| L1210 | Du145 | A2780 | |
| Pt1 | 0.10 ± 0.01 | 0.08 ± 0.05 | 0.27 ± 0.03 |
| Pt1’ | 1.5 ± 0.1 | 0.79 ± 0.08 | 2.7 ± 0.07 |
| Pt2 | 0.009 ± 0.002 | 0.007 ± 0.002 | 0.030 ± 0.004 |
| Pt2’ | 0.46 ± 0.01 | 0.41 ± 0.04 | 1.1 ± 0.1 |
| Pt3 | 0.19 ± 0.01 | 0.44 ± 0.06 | 2.0 ± 0.1 |
| Pt3′ | 0.8 ± 0.2 | 2.7 ± 0.2 | 6.5 ± 0.0 |
| Pt4 | 1.3 ± 0.4 | 2.2 ± 0.1 | 3.7 ± 0.4 |
| Pt4′ | 6 ± 2 | 3 ± 1 | 2.0 ± 0.1 |
| Pt5 | 0.6 ± 0.2 | 1.3 ± 0.4 | 2.6 ± 0.2 |
| Pt5′ | 5.5 ± 0.1 | n.d. | n.d |
| Pt6 | 0.36 ± 0.02 | 0.12 ± 0.03 | 1.1 ± 0.3 |
| Pt6’ | 1.8 ± 0.0 | 1.5 ± 0.03 | 5.6 ± 0.5 |
| Cisplatin | 0.35–1 [a] | 1.2 ± 0.1 | 1.0 ± 0.1 |
| Carboplatin | n.d. | 2.9 ± 0.4 | 0.16 ± 0.0 |
| Oxaliplatin | n.d. | 15 ± 1 | 9 ± 3 |
The most promising analogue from this group of complexes is Pt2 which exhibits over 160-fold greater activity than cisplatin in various cell lines (Table 1). To rationalise such a large difference in cytotoxicity, a comparative transcriptomics approach was undertaken between Pt2 and cisplatin to distinguish the regulation of molecular pathways using the model organism Saccharomyces cerevisiae (yeast) [28,29]. Distinct differences were observed between treatment with Pt2 and cisplatin at a molecular level, with stark contrasts in the up- and down-regulation of numerous molecular pathways. The sulphur-assimilation pathway was shown to be suppressed by Pt2 while cisplatin caused an up-regulation of this pathway; this would subsequently result in increased production of thiol containing biomolecules such as thioredoxins and glutathione, which is thought to mediate resistance as it can deactivate PCs [29,30]. Additionally, genes regulating iron and copper transport across the cell membrane were significantly up-regulated by Pt2 compared to cisplatin. The stark differences observed between Pt2 and cisplatin in altering molecular pathways may partially account for their differences in cytotoxicity.
Despite Pt2 exhibiting potent cytotoxicity in vitro, this activity has not yet translated into in vivo studies. BD-IX rats with peritoneal carcinomatosis (induced by intraperitoneal rat PROb colon cell inoculation) were treated with Pt2, via intravenous and intraperitoneal methods; this treatment did not elicit a tumour suppression response [32]. Furthermore, at pharmacological doses, Pt2 seemed to cause nephrotoxicity [32]. However, in a separate study, the efficacy of Pt1 was compared to cisplatin in female Specific Pathogen Free Swiss nude mice bearing PC3 (human prostate carcinoma) tumour xenografts [33]. Mice treated with either Pt1 or cisplatin demonstrated a comparable decrease in mean tumour weight in relation to the control group. Furthermore, no obvious signs of toxicity were observed in mice treated with Pt1, while half of the cisplatin-treated mice perished by Day 20 [33].
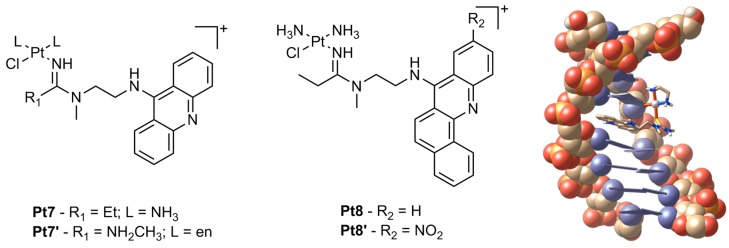
Another promising class of platinum anticancer agents are composed of variations of N-[2-(acridin-9-ylamino)ethyl]-N-methylacetimidamide, linked by a chain of varying length to ligands conjugated around the platinum centre (Figure 5). These complexes have demonstrated exceptional cytotoxicity with Pt7 exhibiting IC50 values down to nanomolar concentrations in non-small cell lung cancer (NSCLC) cell lines (Table 2) [34]. This activity has been attributed to the unique hybrid of DNA binding by these complexes which utilize both intercalation and nonfunctional adduct formation, which are more disruptive than those formed by cisplatin. The acridine moiety is able to intercalate whilst the platinum metal forms a monofunctional adduct with DNA adjacent to the intercalation site [35]. These lesions inhibit DNA synthesis through stalled replication forks and DNA double-strand breaks [35]. Furthermore, these adducts inhibit RNA polymerase II-mediated transcription more prominently than compared to cross-links by cisplatin [36].
Figure 5.
General structure of platinum complexes incorporating acridine and benz[c]acridine (left) and the acridine complex [PtCl(en)(1-{2-(acridin-9-ylamino)ethyl}-1,3-dimethylthiourea)](NO3)2 bound to DNA, as determined through a solution structure (PDB 1XRW) [39]. Counter-ions have been omitted for clarity and en = ethylenediamine. Oxygen is orange, phosphorous is yellow, carbon is cream/white and nitrogen is blue/purple. The base pairs are also represented as blue/purple rectangular panels.
Table 2.
In vitro cytotoxicity of Pt7, Pt7’, Pt8 and Pt8’ against human NSCLC cell lines and HL-60 leukaemia cells. Values taken from references [34,38].
| Complex | IC50 (µM) | ||||
|---|---|---|---|---|---|
| Cell Line | |||||
| NCI-H460 | NCI-H520 | NCI-H522 | A549 | HL-60 | |
| Pt7 | 0.0052 ± 0.0001 | 0.043 ± 0.004 | 0.010 ± 0.001 | 0.0065 ± 0.0002 | – |
| Pt7’ | – | – | – | – | 0.13 |
| Pt8 | 0.24 ± 0.01 | 0.52 ± 0.01 | 0.12 ± 0.02 | 0.32 ± 0.06 | – |
| Pt8’ | 2.4 ± 0.5 | 2.2 ± 0.1 | 3.62 ± 0.08 | 12.4 ± 0.9 | – |
Despite these platinum-acridine complexes exhibiting excellent in vitro cytotoxicity, in vivo studies have revealed severe unwanted toxicities in mice with xenografted NCI-H460 tumours. Although tumour growth was slowed, platinum levels were higher in healthy tissue than they were in the tumour, with the possibility of hepatotoxicity or nephrotoxicity as a result [37]. Hence variations of the original complex were synthesised in which the intercalating moiety was substituted with benz[c]acridine to increase its size and hydrophobicity (Figure 5, Pt8) [34]. Pt8 was reported to be slightly less cytotoxic than Pt7 (Table 2) although it was found to have significantly different cellular pharmacology and target binding properties, which may result in a more favourable therapeutic window in vivo.
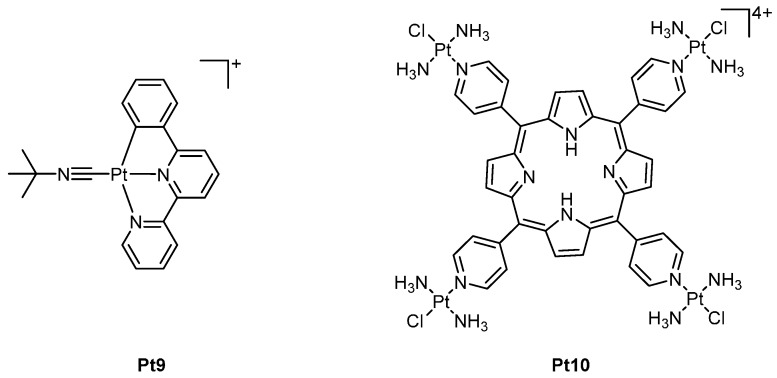
More recently, a new class of luminescent cyclometalated PCs have been reported consisting of 6-phenyl-2,2’-bipyridyl (Figure 6). These complexes have demonstrated the ability to form emissive exciplexes with DNA via intercalation [40]. The most cytotoxic complex from this class of compounds, Pt9, exhibits in vitro IC50 values of 0.009 and 0.010 µM against oral epidermal carcinoma (KB) and neuroblastoma (SH-5YSY), respectively [40]. DNA damage by Pt9, resulting from its ability to stabilise the Topoisomerase I-DNA complex, has been attributed to its potent anticancer activity. Subsequent in vivo testing in nude mice implanted with NCI-460 showed tumour inhibition by 60% with no reported side-effects [40].
Figure 6.
Structures of the luminescent cyclometalated PC, Pt9 and the tetraplatinated porphyrin, Pt10. Counter-ions have been omitted for clarity.
Platinated porphyrins are another class of complexes that have shown very promising results and have the potential to be activated via light irradiation (Figure 6). Fluorescence imaging experiments utilising the lead compound in this series Pt10, revealed the compound selectively localised within the nucleus. Pt10 is reported to interact with DNA via a dual binding mode involving intercalation and, to a lesser extent, covalent binding through the platinum centres [41]. DNA photocleavage experiments showed no damage to DNA in the absence of light, however upon irradiation, photocleavage of DNA was markedly enhanced [41]. In vitro studies of Pt10 in cisplatin-resistant human ovarian cancer (CP70) cell lines showed IC50 values of >100 µM in the absence of light, however, when irradiated (420 nm, 6.95 J·cm−2), this IC50 value decreased significantly to 0.019 µM [41].
3. Copper
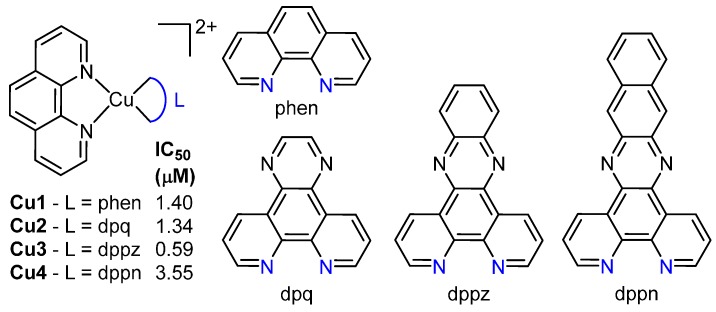
Copper has a long history in medicinal inorganic chemistry, particularly in antibacterial and anticancer agents, due to its natural bioavailability, its role in angiogenesis and increased uptake in cancerous tissues [42,43]. The role of copper in the growth of tumours is significant enough that copper capturing agents have progressed to phase II clinical trials [44,45]. Copper complexes most often initiate their cytotoxic effect through oxygen-dependent or -independent DNA cleavage. Here, DNA intercalation can assist the cleavage process by allowing close proximity of copper complexes to the double strand [46,47,48]. Copper is also capable of a large variety of coordination geometries, often producing very different species from reactions with very similar starting reagents[46,47]. The most well-known types of copper nucleases are those incorporating phen such as [Cu(phen)2]2+. In a recent study, this scaffold was modified to afford additional complexes of the type [Cu(phen)(L)], where L is one of phen (Cu1), dpq (Cu2), dppz (Cu3) or benzo[i]dppz (dppn, Cu4, Figure 7) [49]. Intercalation was theorised to occur within both the major and minor grooves; Cu2 and Cu3 demonstrated 60-hold higher CT-DNA binding affinity than Cu1 with binding constants of approximately 3 × 107 M. All complexes could cleave plasmid DNA through an oxidative mechanism, and each exhibited low-micromolar activity against SKOV3 human cancer cells (Figure 7).
Figure 7.
Structures of copper complexes Cu1–4, and the IC50 value of each complex in the SKOV3 human cancer cell line. Blue-coloured atoms are those that coordinate to the copper centre for each L example. Counter-ions have been omitted for clarity.
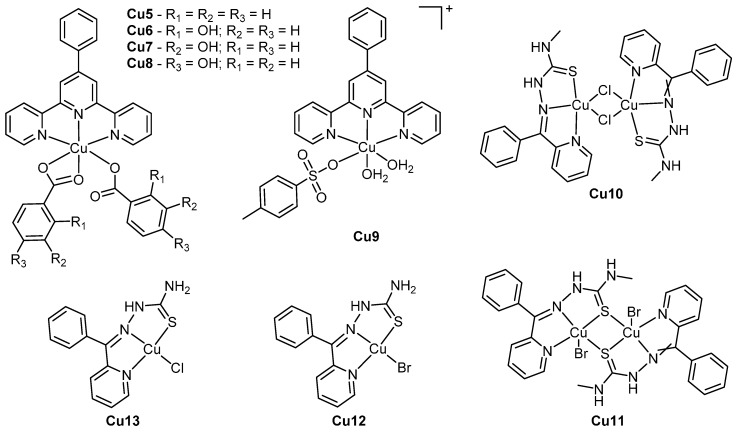
The scope of copper nucleases expands far beyond copper phenanthrenes. For example a recent series of complexes of the type [Cu(4phterpy)(L)2] or [Cu(4phterpy)(L)(H2O)2](L) (where 4phterpy is 4’-phenyl-2,2’:6’,2”-terpyridine and L is one of p-toluenesulphonate, benzoate or o-, m- or p-hydroxybenzoate, Cu5–9, Figure 8) were found to be cytotoxic to HCT116 colorectal carcinoma and HepG2 hepatocellular carcinoma cells while exhibiting lower activity in normal human fibroblasts (Table 3) [48]. The model complex Cu9 induced apoptosis in HCT116 cells in a caspase-3 related mechanism; the higher cytotoxicity of Cu9 relative to the others was theorised to be due to its labile aqua ligands and charged nature, which could encourage cellular uptake by human copper transporters [50]. All complexes intercalated with DNA, exhibiting binding constants of 105–106 M−1, and each was capable of hydrolytically cleaving plasmid DNA under both aerobic and anaerobic conditions in a radical and oxygen-independent manner. Cu6 produced a substantial amount of linear DNA during cleavage experiments relative to Cu5, Cu7 and Cu8, suggesting that the ortho position of the benzoate hydroxyl group was optimal for DNA cleavage.
Figure 8.
Structures of complexes Cu5–13. Counter-ions have been omitted for clarity.
Table 3.
In vitro cytotoxicity of complexes Cu5–13 in various cell lines, expressed as IC50 values with standard error (1 significant figure).
| Complex | IC50 (μM) | Reference | ||
|---|---|---|---|---|
| HCT116 | HepG-2 | NHF [a] | ||
| Cu5 | 0.31 ± 0.03 | 14.0 ± 0.5 | >20 | [48] |
| Cu6 | 0.468 ± 0.006 | 13.6 ± 0.5 | >20 | |
| Cu7 | 0.44 ± 0.09 | 0.54 ± 0.03 | >5 | |
| Cu8 | 1.5 ± 0.2 | 0.7 ± 0.1 | >5 | |
| Cu9 | 0.07 ± 0.05 | 0.24 ± 0.02 | 5.483 ± 0.003 | |
| Complex | HeLa | HepG-2 | NCI-H460 | Reference |
| Cu10 | 0.16 ± 0.05 | 0.10 ± 0.04 | 0.08 ± 0.01 | [47] |
| Cu11 | 0.59 ± 0.02 | 0.20 ± 0.01 | 0.16 ± 0.01 | |
| Cu12 | 1.4 ± 0.6 | 1.1 ± 0.4 | 2.0 ± 0.3 | |
| Cu13 | 1.3 ± 0.2 | 0.8 ± 0.2 | 1.5 ± 0.7 | |
[a] NHF = normal human fibroblasts.
Another recent study focused on a series of copper semicarbazone complexes: [Cu(Bp4mT)(μ-Cl)]2 (Cu10), [Cu(μ-Bp4mT)Br]2 (Cu11), [Cu(HBpT)Cl] (Cu12), and [Cu(HBpT)Br] (Cu13) (where Bp4mT is 2-benzoylpyridine-4-methylthiosemicarbazone and HBpT is 2-benzoylpyridinethiosemicarbazone, (Figure 8) [47]. Each complex is capable of intercalation and DNA cleavage through an oxidative mechanism involving hydroxide radicals and singlet oxygen. All complexes were found to be at least ten times more active than cisplatin against HeLa, HepG-2 and NCI-H460 cells, achieving IC50 values as low as 0.08 ± 0.01 μM (Table 3). The dinuclear complexes Cu10 and Cu11 were more than twice as active as the mononuclear Cu12 and Cu13; and it was proposed that the increased lipophilicity afforded by the methylated nitrogen of the Bp4mT ligand could increase the passive diffusion of Cu10 and Cu11 into cancerous cells. Alternatively, it could also be as a consequence of double the quantity of active components in the dimer.
There are many other notable recent studies of intercalating copper nucleases [46,51,52,53,54]. Two copper intercalators of (2-((quinolin-8-ylimino)methyl)pyridine) recently exhibited activity against HeLa, MCF-7 and A549 cells and cleaved DNA without addition of peroxide [46]. A bis-thiosemicarbazone copper complex was found to intercalate and cleave DNA, exhibit micromolar-level activity against HCT116 cells, and induce augmented tumour regression in a murine HCT116 cell xenograft model. However, the DNA binding and biological activity were not necessarily correlated for all complexes in the study [51]. Overall, copper intercalators have demonstrated potential as anticancer agents due to their efficient DNA binding and cleavage activity.
4. Ruthenium
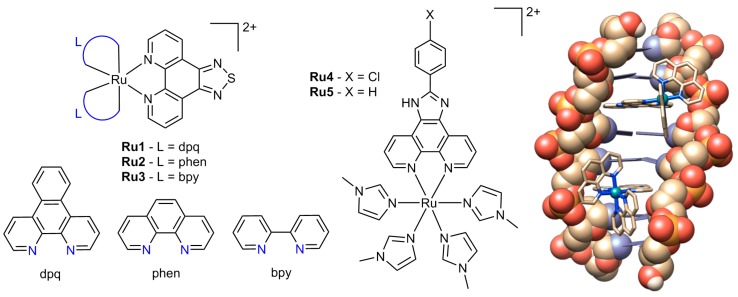
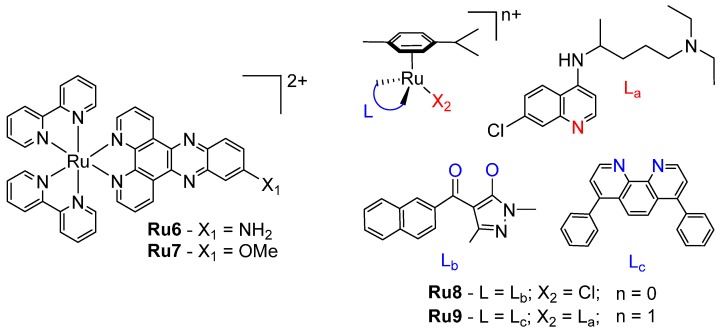
The octahedral geometry and interchangeable oxidation states of Ru(II) and Ru(III) allow for a large diversity of ligand combinations, and the inherent fluorescent properties and high kinetic stability of ruthenium compounds are extremely beneficial to biological studies [55], as well as the design of photo-activated complexes [56]. Some ruthenium anticancer complexes have advanced to clinical trials, with the complex indazolium trans-[tetrachlorobis(1H-indazole)ruthenate(III)] (KP1019) successfully completing phase I clinical trials [57], whereas the complex (ImH)[trans-RuCl4(DMSO)(Im)] (NAMI-A, where Im = imidazole, DMSO = dimethylsulphoxide) has progressed to phase II clinical trials [58]. The most common type of ruthenium intercalators are polypyridyl complexes [Ru(L1)2(L2)]2+, in which L2 is a long intercalating ligand such as dppz or dppn and L1 are two ancillary ligands that can affect DNA binding properties (Figure 6) [59]. While these types of complexes often display low levels of cytotoxicity, there are several polypyridyl compounds recently synthesised that demonstrate potent activity. A recent series of complexes of the type [Ru(L)2(tdzp)]2+ (where L is one of bpy, phen or dpq and tdzp is [1,2,5]-thiadiazolo-[3,4-f]-[1,10]-phenanthroline, Ru1–3, Figure 9) were found to intercalate with DNA with binding constants of 103–104 M−1 in a manner dependent on the planarity of ligand. These complexes accumulated within the nuclei of cells and were antiproliferative against HeLa cells (Table 4) [60]. In a different study, complexes of the type [Ru(MeIm)4(L)]2+ (where MeIm = 1-methylimidazol and L = 2-(4-chlorophenyl)-1H-imidazo[4,5-f] [1,10]phenanthroline, Ru4, or 2-phenyl-1H-imidazo[4,5-f] [1,10]phenanthroline, Ru5, Figure 9) were shown to intercalate with DNA and cause cell cycle arrest in the A549 cell line at G0/G1 phase.The complexes also induced mitochondrial dysfunction and ultimately apoptosis involving ROS accumulation and Bcl-2 and caspase family activation. The cytotoxicity of each complex was comparable with cisplatin in several cell lines, with Ru4 being more active than Ru5 (Table 4) [61].
Figure 9.
Chemical structures of ruthenium polypyridyl complexes Ru1–5 (left) and the X-ray crystal structure of rac-[Ru(phen)2(dppz)]2+ bound to DNA sequence d(ATGCAT)2 (right). The extended aromatic ligand intercalates and separates the DNA base pairs, here shown with both the ∆ and Λ enantiomers bound. Sourced from PDB file 4JD8 [59]. Blue-coloured atoms are those that coordinate to the ruthenium centre in each L example. Counter-ions have been omitted for clarity. Oxygen is orange, phosphorous is yellow, carbon is cream/white and nitrogen is blue/purple. The base pairs are also represented as blue/purple rectangular panels.
Table 4.
In vitro cytotoxicity of complexes Ru1–7 in HeLa cells, expressed as IC50 values with standard error (1significant figure). Cisplatin is included as a reference.
| Complex | IC50 (µM) | Reference | Complex | IC50 (µM) | Reference |
|---|---|---|---|---|---|
| Ru1 | 28.0 ± 0.1 | [60] | Ru6 [a] | 2.0 ± 0.9 | [62] |
| Ru2 | 21.00 ± 0.08 | Ru7 [a] | 5.5 ± 0.7 | ||
| Ru3 | 19.00 ± 0.08 | – | – | – | |
| Ru4 | 27 ± 2 | [61] | Cisplatin | 15 ± 2 | [61] |
| Ru5 | 25 ± 2 |
[a] Values account for irradiation at 420 nm.
Polypyridyl ruthenium complexes have also been used as intercalating photodynamic agents [63,64]. Complexes of the type [Ru(bpy)2(R-dppz)]2+ (where R is either NH2, Ru6 or OMe, Ru7, Figure 10) intercalated with DNA via the R-dppz ligand and achieved phototoxic indices of >150 and 42, respectively, against HeLa cells when irradiated with light at 420 nm (Table 4) [62]. A polypyridyl ruthenium complex incorporating an appended anthracene demonstrated photocleavage of DNA through both ruthenium-derived singlet oxygen and anthracene-derived radicals, as well as light-induced cytotoxicity in F98 glioma cells [65,66]. Ruthenium arene “piano-stool” complexes are very prominent anticancer agents, and some studies have reported a dual-binding mode in which ruthenation occurs through a leaving group while the p-cymene group intercalates between nearby bases [67]. Recent examples include a series of complexes incorporating 1,3-Dimethyl-4-acylpyrazolon-5-ato ligands, of which the lead compound, [Ru(p-cymene)(1,3-dimethyl-4-(1-naphthoyl)-pyrazolon-5-ate)Cl] (Ru8, Figure 10), demonstrated potent activity against several cell lines [67], and a chloroquine-tethered complex with submicromolar activity against A549 and MCF-7 cells (Ru9, Figure 10) [68].
Figure 10.
Chemical structures of ruthenium arene complexes Ru6–9. Counter-ions have been omitted for clarity. Ligands with a blue label coordinate at the “L” position of the arene through the blue-coloured oxygen or nitrogen atoms. La coordinates at the X2 position through the red nitrogen.
5. Other Metals
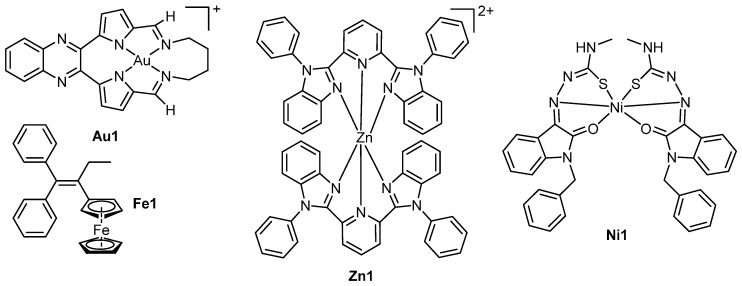
A large spectrum of transition metal complexes have been used as anticancer agents, although for many intercalation is not required for activity. Nonetheless some recent examples of less common transition metal intercalators have emerged in the literature. For example, gold complexes have been relatively successful in medicinal chemistry [69,70,71], although most active anticancer complexes do not target DNA [72,73,74]. Recently reported macrocyclic gold(III) complexes that incorporated a quinoxaline moiety to promote DNA intercalation demonstrated cytotoxic activity [75]. The lead compound, [Au(12,13,14,15-tetrahydro-6,9:18,21-diepimino[1,6]diazacycloctadecino[12,13-b]quinoxaline)]+ (Au1, Figure 11) exhibited low micromolar activity in a panel of human cell lines, particularly in leukaemia and central nervous system cancers, and was well-tolerated by nude tumour-less mice at high doses. Enzyme inhibition assays, molecular modelling and surface plasmon resonance studies revealed that Au1 was an inhibitor of human topoisomerase 1 (Top1). Here inhibition occurred through intercalative binding to the DNA substrate of Top1 and not through binding to Top1 itself. Another study focused on gold(I) complexes consisting of a DNA intercalating 1,8-naphthalimide tethered to a gold centre through an N-heterocyclic carbene moiety [76]. The complexes were designed as dual-action anticancer agents that both intercalate with DNA and inhibit thioredoxin reductase activity; low micromolar activity was exhibited by the four complexes in HT-29 and MCF-7 cell lines.
Figure 11.
Structures of the metallointercalators Au1, Fe1, Zn1 and Ni1. Counter-ions have been omitted for clarity.
Nickel and zinc have also seen widespread use in medicinal chemistry as DNA nucleases due to their natural abundance in humans and important roles in cellular functions [77,78]. A recent zinc study focused upon complexes of 2,6-bis(1-phenyl-1H-benzo[d]imidazol-2-yl)pyridine (bpbp) [77]. The lead compound [Zn(bpbp)2]2+ (Zn1, Figure 11) demonstrated low micromolar activity in a variety of cell lines, with in IC50 of 2.9 ± 0.3 μM against MCF-7 cells. The hypothesised mechanism of action was DNA damage via intercalation and cleavage, resulting in elevated levels of phosphorylated p53 gene and apoptosis. Another intercalating zinc complex of 5-bromo-8-hydroxyquinoline displayed higher cytotoxicity in BEL-7404 and T-24 cells than cisplatin and induced cell cycle arrest in the G2 phase of the BEL-7404 cells [79]. A series of nickel isotin thiosemicarbazone complexes were also found to intercalate with DNA and achieve up to 99.8% cleavage of plasmid DNA without the addition of peroxide [78]. The lead compound, [Ni(L)2] (where L = (Z)-2-(1-benzyl-2-oxoindolin-3-ylidene)-N-methylhydrazinecarbothioamide, Ni1, Figure 11) was highly active against MCF7 cells with an IC50 value of <0.1 μM. In an additional study, nickel and cobalt complexes of the anaesthetic lidocaine produced low micromolar IC50 values against a panel of human cell lines. Here the nickel complexes were generally more active than the cobalt [80]. All complexes in the study cleaved plasmid DNA in the presence of H2O2 in a singlet oxygen-involved method.
Iron has also been exploited in the development of anticancer agents as it is an essential component of various biological processes including erythropoiesis, electron transport and DNA synthesis [81]. Additionally, iron most commonly exists in two oxidation states (Fe(II) and (Fe(III)), which allows it to participate in important redox reactions [82]. A recent series of iron-based complexes that incorporate ferrocene as part of a modified tamoxifen base structure have been reported (Figure 11). The lead compound, Fe1, showed no activity against non-cancerous glomerular basement membrane (GBM) monkey cells, however exhibited low micromolar activity in cancerous cell lines with IC50 values of 0.9 and 1.04 µM against human colon cancer (HCT-8) and acute promyelocytic leukaemia (HL-60), respectively [83]. Mechanistic studies revealed the activation of caspases 3 and 7, externalisation of phosphatidylserine and increased DNA fragmentation, which were attributed to an intercalative mode of binding of Fe1 with dsDNA and ssDNA [83].
6. Conclusions
Over the past five decades, an extensive catalogue of metal complexes have been synthesised and evaluated as potential anticancer agents, with the primary aim of overcoming the drawbacks of currently used metallodrugs. Transition metal intercalators have been studied since the 1970s as alternatives that exerts cytotoxicity through different modes of action to cisplatin-like chemotherapeutics. A variety of transition metal-based intercalators have been reviewed here, all of which are potently cytotoxic and demonstrate DNA intercalation. Platinum intercalators can kill cancerous cells via unconventional methods at nanomolar concentrations, and some have also demonstrated some tumour-inhibition results in vivo. Copper complexes can intercalate and cleave DNA, with recent studies reporting potent compounds both with the phenanthrene architype coordination and without. Nickel and zinc intercalators are also proven to be efficient DNA nucleases. Ruthenium polypyridyl and arene complexes have exhibited micromolar activity against several human cell lines and some have proven potential as photoactivated drugs. Recent pairing of intercalating ligands with other metals such as gold and iron has produced even more cytotoxic complexes with distinctly different mechanisms to kill cancerous cells. The huge variety of transition metal properties and ligand combinations has produced an extremely broad spectrum of intercalating anticancer complexes, each with a unique mechanism of action. The continued expansion of this spectrum has great potential to reveal metallointercalators which can outperform current metallodrugs and provide more effective chemotherapy.
Acknowledgments
The authors would like to thank Western Sydney University for financial support through internal research grants. Krishant M. Deo, Benjamin J. Pages and Dale L. Ang were supported by an Australian Postgraduate Award. Benjamin J. Pages and Dale L. Ang were additionally supported by a Western Sydney University Top-Up Award. We also thank Samuel J. Frost for the contribution of the graphical abstract.
Author Contributions
Krishant M. Deo wrote the “Introduction” and “Platinum” sections, as well as the iron portion of “Other Metals”. Benjamin J. Pages organised the content of the paper and wrote the “Copper” and “Other Metals” section, aside from the iron portion. Dale L. Ang researched and rendered several versions of the DNA PDB files, and contributed to the “Ruthenium” section. Christopher P. Gordon extensively revised the paper and provided many helpful suggestions regarding figure and paragraph layout as well as spelling and grammar checks. Janice R. Aldrich-Wright fulfilled similar duties to Christopher and also contributed to the “Ruthenium” section.
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Rosenberg B., van Camp L., Krigas T. Inhibition of cell division in Escherichia coli by electrolysis products from a platinum electrode. Nature. 1965;205:698–699. doi: 10.1038/205698a0. [DOI] [PubMed] [Google Scholar]
- 2.Loehrer P.J., Einhorn L.H. Drugs five years later. Cisplatin. Ann. Intern. Med. 1984;100:704–713. doi: 10.7326/0003-4819-100-5-704. [DOI] [PubMed] [Google Scholar]
- 3.Provencher-Mandeville J., Debnath C., Mandal S.K., Leblanc V., Parent S., Asselin É., Bérubé G. Design, synthesis and biological evaluation of estradiol-PEG-linked Platinum(II) hybrid molecules: Comparative molecular modeling study of three distinct families of hybrids. Steroids. 2011;76:94–103. doi: 10.1016/j.steroids.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 4.Cepeda V., Fuertes M.A., Castilla J., Alonso C., Quevedo C., Pérez J.M. Biochemical mechanisms of cisplatin cytotoxicity. Anti-Cancer Agents Med. Chem. 2007;7:3–18. doi: 10.2174/187152007779314044. [DOI] [PubMed] [Google Scholar]
- 5.Florea A.M., Büsselberg D. Cisplatin as an anti-tumor drug: Cellular mechanisms of activity, drug resistance and induced side effects. Cancers. 2011;3:1351–1371. doi: 10.3390/cancers3011351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Johnstone T.C., Wilson J.J., Lippard S.J. Monofunctional and higher-valent platinum anticancer agents. Inorg. Chem. 2013;52:12234–12249. doi: 10.1021/ic400538c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cleare M.J., Hoeschele J.D. Studies on the antitumor activity of group VIII transition metal complexes. Part I. Platinum (II) complexes. Bioinorg. Chem. 1973;2:187–210. doi: 10.1016/S0006-3061(00)80249-5. [DOI] [Google Scholar]
- 8.Lovejoy K.S., Lippard S.J. Non-traditional platinum compounds for improved accumulation, oral bioavailability, and tumor targeting. Dalton Trans. 2009;48:10651–10659. doi: 10.1039/b913896j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lin C., Mathad R.I., Zhang Z., Sidell N., Yang D. Solution structure of a 2:1 complex of anticancer drug XR5944 with TFF1 estrogen response element: Insights into DNA recognition by a bis-intercalator. Nucleic Acids Res. 2014;42:6012–6024. doi: 10.1093/nar/gku219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gelasco A., Lippard S.J. NMR solution structure of a DNA dodecamer duplex containing a cis-Diammineplatinum(II) d(GpG) intrastrand cross-link, the major adduct of the anticancer drug cisplatin. Biochemistry. 1998;37:9230–9239. doi: 10.1021/bi973176v. [DOI] [PubMed] [Google Scholar]
- 11.Drew H.R., Wing R.M., Takano T., Broka C., Tanaka S., Itakura K., Dickerson R.E. Structure of a B-DNA dodecamer: Conformation and dynamics. Proc. Natl. Acad. Sci. USA. 1981;78:2179–2183. doi: 10.1073/pnas.78.4.2179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lerman L.S. Structural considerations in the interaction of DNA and acridines. J. Mol. Biol. 1961;3:18–30. doi: 10.1016/S0022-2836(61)80004-1. [DOI] [PubMed] [Google Scholar]
- 13.Long E.C., Barton J.K. On demonstrating DNA intercalation. Acc. Chem. Res. 1990;23:271–273. doi: 10.1021/ar00177a001. [DOI] [Google Scholar]
- 14.Garbutcheon-Singh K.B., Myers S., Harper B.W.J., Ng N.S., Dong Q., Xie C., Aldrich-Wright J.R. The effects of 56MESS on mitochondrial and cytoskeletal proteins and the cell cycle in MDCK cells. Metallomics. 2013;5:1061–1067. doi: 10.1039/c3mt00023k. [DOI] [PubMed] [Google Scholar]
- 15.Jennette K.W., Lippard S.J., Vassiliades G.A., Bauer W.R. Metallointercalation reagents. 2-hydroxyethanethiolato(2,2’,2’-terpyridine)-platinum(II) monocation binds strongly to DNA by intercalation. Proc. Natl. Acad. Sci. USA. 1974;71:3839–3843. doi: 10.1073/pnas.71.10.3839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bowler B.E., Lippard S.J. Modulation of platinum antitumor drug binding to DNA by linked and free intercalators. Biochemistry. 1986;25:3031–3038. doi: 10.1021/bi00358a044. [DOI] [PubMed] [Google Scholar]
- 17.Sigman D.S., Graham D.R., D’Aurora V., Stern A.M. Oxygen-dependent cleavage of DNA by the 1,10-phenanthroline. Cuprous complex. Inhibition of Escherichia coli DNA polymerase I. J. Biol. Chem. 1979;254:12269–12272. [PubMed] [Google Scholar]
- 18.Kelly J.M., Tossi A.B., McConnell D.J., OhUigin C. A Study of the interactions of some Polypyridylruthenium(II) complexes with DNA using fluorescence spectroscopy, topoisomerisation and thermal denaturation. Nucleic Acids Res. 1985;13:6017–6034. doi: 10.1093/nar/13.17.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Friedman A.E., Chambron J.C., Sauvage J.P., Turro N.J., Barton J.K. A molecular light switch for DNA: Ru(bpy)2(dppz)2+ J. Am. Chem. Soc. 1990;112:4960–4962. doi: 10.1021/ja00168a052. [DOI] [Google Scholar]
- 20.Kielkopf C.L., Erkkila K.E., Hudson B.P., Barton J.K., Rees D.C. Structure of a photoactive rhodium complex intercalated into DNA. Nat. Struct. Mol. Biol. 2000;7:117–121. doi: 10.1038/72385. [DOI] [PubMed] [Google Scholar]
- 21.Yan Y.K., Melchart M., Habtemariam A., Sadler P.J. Organometallic chemistry, biology and medicine: Ruthenium arene anticancer complexes. Chem. Commun. 2005;38:4764–4776. doi: 10.1039/b508531b. [DOI] [PubMed] [Google Scholar]
- 22.Cohen S.M. New approaches for medicinal applications of bioinorganic chemistry. Curr. Opin. Chem. Biol. 2007;11:115–120. doi: 10.1016/j.cbpa.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 23.Romero-Canelón I., Sadler P.J. Next-generation metal anticancer complexes: Multitargeting via redox modulation. Inorg. Chem. 2013;52:12276–12291. doi: 10.1021/ic400835n. [DOI] [PubMed] [Google Scholar]
- 24.Asadi M., Safaei E., Ranjbar B., Hasani L. Thermodynamic and spectroscopic study on the binding of cationic Zn(II) and Co(II) tetrapyridinoporphyrazines to calf thymus DNA: The role of the central metal in binding parameters. New J. Chem. 2004;28:1227–1234. doi: 10.1039/b404068f. [DOI] [Google Scholar]
- 25.Pages B.J., Li F., Wormell P., Ang D.L., Clegg J.K., Kepert C.J., Spare L.K., Danchaiwijit S., Aldrich-Wright J.R. Synthesis and analysis of the anticancer activity of Platinum(II) complexes incorporating dipyridoquinoxaline variants. Dalton Trans. 2014;43:15566–15575. doi: 10.1039/C4DT02133A. [DOI] [PubMed] [Google Scholar]
- 26.Pages B.J., Zhang Y., Li F., Sakoff J., Gilbert J., Aldrich-Wright J.R. Cytotoxicity and structural analyses of 2,2′-Bipyridine-, 4,4′-Dimethyl-2,2′-bipyridine- and 2-(2′-Pyridyl)quinoxalineplatinum(II) complexes. Eur. J. Inorg. Chem. 2015;2015:4167–4175. doi: 10.1002/ejic.201500754. [DOI] [Google Scholar]
- 27.Pages B.J., Sakoff J., Gilbert J., Rodger A., Chmel N.P., Jones N.C., Kelly S.M., Ang D.L., Aldrich-Wright J.R. Multifaceted studies of the DNA interactions and in vitro cytotoxicity of anticancer polyaromatic Platinum(II) complexes. Chem. Eur. J. 2016;22:8943–8954. doi: 10.1002/chem.201601221. [DOI] [PubMed] [Google Scholar]
- 28.Wang S., Higgins V., Aldrich-Wright J., Wu M. Identification of the molecular mechanisms underlying the cytotoxic action of a potent platinum metallointercalator. J. Chem. Biol. 2012;5:51–61. doi: 10.1007/s12154-011-0070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S., Wu M.J., Higgins V.J., Aldrich-Wright J.R. Comparative analyses of cytotoxicity and molecular mechanisms between platinum metallointercalators and cisplatin. Metallomics. 2012;4:950–959. doi: 10.1039/c2mt20102j. [DOI] [PubMed] [Google Scholar]
- 30.Kemp S., Wheate N.J., Pisani M.J., Aldrich-Wright J.R. Degradation of bidentate-coordinated platinum(II)-based DNA intercalators by reduced l-glutathione. J. Med. Chem. 2008;51:2787–2794. doi: 10.1021/jm7016072. [DOI] [PubMed] [Google Scholar]
- 31.Wheate N.J., Taleb R.I., Krause-Heuer A.M., Cook R.L., Wang S., Higgins V.J., Aldrich-Wright J.R. Novel Platinum(II)-based anticancer complexes and molecular hosts as their drug delivery vehicles. Dalton Trans. 2007;43:5055–5064. doi: 10.1039/b704973k. [DOI] [PubMed] [Google Scholar]
- 32.Moretto J., Chauffert B., Ghiringhelli F., Aldrich-Wright J.R., Bouyer F. Discrepancy between in vitro and in vivo antitumor effect of a new Platinum(II) metallointercalator. Investig. New Drug. 2011;29:1164–1176. doi: 10.1007/s10637-010-9461-z. [DOI] [PubMed] [Google Scholar]
- 33.Fisher D.M., Fenton R.R., Aldrich-Wright J.R. In vivo studies of a Platinum(II) metallointercalator. Chem. Commun. 2008;43:5613–5615. doi: 10.1039/b811723c. [DOI] [PubMed] [Google Scholar]
- 34.Pickard A.J., Liu F., Bartenstein T.F., Haines L.G., Levine K.E., Kucera G.L., Bierbach U. Redesigning the DNA-targeted chromophore in platinum–acridine anticancer agents: A structure-activity relationship study. Chem. Eur. J. 2014;20:16174–16187. doi: 10.1002/chem.201404845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheung-Ong K., Song K.T., Ma Z., Shabtai D., Lee A.Y., Gallo D., Heisler L.E., Brown G.W., Bierbach U., Giaever G., et al. Comparative chemogenomics to examine the mechanism of action of DNA-targeted platinum-acridine anticancer agents. ACS Chem. Biol. 2012;7:1892–1901. doi: 10.1021/cb300320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kostrhunova H., Malina J., Pickard A.J., Stepankova J., Vojtiskova M., Kasparkova J., Muchova T., Rohlfing M.L., Bierbach U., Brabec V. Replacement of a thiourea with an amidine group in a monofunctional platinum–acridine antitumor agent. Effect on DNA interactions, DNA adduct recognition and repair. Mol. Pharm. 2011;8:1941–1954. doi: 10.1021/mp200309x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martins E.T., Baruah H., Kramarczyk J., Saluta G., Day C.S., Kucera G.L., Bierbach U. Design, Synthesis, and biological activity of a novel non-cisplatin-type platinum−acridine pharmacophore. J. Med. Chem. 2001;44:4492–4496. doi: 10.1021/jm010293m. [DOI] [PubMed] [Google Scholar]
- 38.Baruah H., Wright M.W., Bierbach U. Solution structural study of a DNA duplex containing the Guanine-N7 adduct formed by a cytotoxic platinum−acridine hybrid agent. Biochemistry. 2005;44:6059–6070. doi: 10.1021/bi050021b. [DOI] [PubMed] [Google Scholar]
- 39.Ma Z., Choudhury J.R., Wright M.W., Day C.S., Saluta G., Kucera G.L., Bierbach U. A non-cross-linking platinum−acridine agent with potent activity in non-small-cell lung cancer. J. Med. Chem. 2008;51:7574–7580. doi: 10.1021/jm800900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zou T., Liu J., Lum C.T., Ma C., Chan R.C.T., Lok C.N., Kwok W.M., Che C.M. Luminescent cyclometalated Platinum(II) complex forms emissive intercalating adducts with double-stranded DNA and RNA: Differential emissions and anticancer activities. Angew. Chem. Int. Ed. 2014;53:10119–10123. doi: 10.1002/anie.201405384. [DOI] [PubMed] [Google Scholar]
- 41.Naik A., Rubbiani R., Gasser G., Spingler B. Visible-light-induced annihilation of tumor cells with platinum–porphyrin conjugates. Angew. Chem. 2014;126:7058–7061. doi: 10.1002/ange.201400533. [DOI] [PubMed] [Google Scholar]
- 42.Finney L., Vogt S., Fukai T., Glesne D. Copper and angiogenesis: Unravelling a relationship key to cancer progression. Clin. Exp. Pharmacol. Physiol. 2009;36:88–94. doi: 10.1111/j.1440-1681.2008.04969.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wende C., Lüdtke C., Kulak N. Copper complexes of N-donor ligands as artificial nucleases. Eur. J. Inorg. Chem. 2014;2014:2597–2612. doi: 10.1002/ejic.201400032. [DOI] [Google Scholar]
- 44.Brewer G.J., Dick R.D., Grover D.K., LeClaire V., Tseng M., Wicha M., Pienta K., Redman B.G., Jahan T., Sondak V.K., et al. Treatment of metastatic cancer with tetrathiomolybdate, an anticopper, antiangiogenic agent: Phase I study. Clin. Cancer Res. 2000;6:1–10. [PubMed] [Google Scholar]
- 45.Pass H.I., Brewer G.J., Dick R., Carbone M., Merajver S. A Phase II trial of tetrathiomolybdate after surgery for malignant mesothelioma: Final results. Ann. Thorac. Surg. 2008;86:383–390. doi: 10.1016/j.athoracsur.2008.03.016. [DOI] [PubMed] [Google Scholar]
- 46.Lu J., Sun Q., Li J.L., Jiang L., Gu W., Liu X., Tian J.L., Yan S.P. Two water-soluble Copper(II) complexes: Synthesis, characterization, DNA cleavage, protein binding activities and in vitro anticancer activity studies. J. Inorg. Biochem. 2014;137:46–56. doi: 10.1016/j.jinorgbio.2014.03.015. [DOI] [PubMed] [Google Scholar]
- 47.Liu Y.H., Li A., Shao J., Xie C.Z., Song X.Q., Bao W.G., Xu J.Y. Four Cu(II) complexes based on antitumor chelators: Synthesis, structure, DNA binding/damage, HSA interaction and enhanced cytotoxicity. Dalton Trans. 2016;45:8036–8049. doi: 10.1039/C6DT00451B. [DOI] [PubMed] [Google Scholar]
- 48.Ma Z., Zhang B., Guedes da Silva M.F.C., Silva J., Mendo A.S., Baptista P.V., Fernandes A.R., Pombeiro A.J.L. Synthesis, Characterization, thermal properties and antiproliferative potential of Copper(II) 4′-phenyl-terpyridine compounds. Dalton Trans. 2016;45:5339–5355. doi: 10.1039/C5DT02744F. [DOI] [PubMed] [Google Scholar]
- 49.Molphy Z., Prisecaru A., Slator C., Barron N., McCann M., Colleran J., Chandran D., Gathergood N., Kellett A. Copper phenanthrene oxidative chemical nucleases. Inorg. Chem. 2014;53:5392–5404. doi: 10.1021/ic500914j. [DOI] [PubMed] [Google Scholar]
- 50.Gupta A., Lutsenko S. Human copper transporters: Mechanism, role in human diseases and therapeutic potential. Future Med. Chem. 2009;1:1125–1142. doi: 10.4155/fmc.09.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Palanimuthu D., Shinde S.V., Somasundaram K., Samuelson A.G. In vitro and in vivo anticancer activity of copper bis(thiosemicarbazone) complexes. J. Med. Chem. 2013;56:722–734. doi: 10.1021/jm300938r. [DOI] [PubMed] [Google Scholar]
- 52.Zhou X.Q., Li Y., Zhang D.Y., Nie Y., Li Z.J., Gu W., Liu X., Tian J.L., Yan S.P. Copper complexes based on chiral schiff-base ligands: DNA/BSA binding ability, DNA cleavage activity, cytotoxicity and mechanism of apoptosis. Eur. J. Med. Chem. 2016;114:244–256. doi: 10.1016/j.ejmech.2016.02.055. [DOI] [PubMed] [Google Scholar]
- 53.Lian W.J., Wang X.T., Xie C.Z., Tian H., Song X.Q., Pan H.T., Qiao X., Xu J.Y. Mixed-ligand Copper(II) schiff base complexes: The role of the co-ligand in DNA binding, DNA cleavage, protein binding and cytotoxicity. Dalton Trans. 2016;45:9073–9087. doi: 10.1039/C6DT00461J. [DOI] [PubMed] [Google Scholar]
- 54.Meenongwa A., Brissos R.F., Soikum C., Chaveerach P., Gamez P., Trongpanich Y., Chaveerach U. Effects of N,N-heterocyclic ligands on the in vitro cytotoxicity and DNA interactions of Copper(II) chloride complexes from amidino-O-methylurea ligands. New J. Chem. 2016;40:5861–5876. doi: 10.1039/C5NJ03439F. [DOI] [Google Scholar]
- 55.Puckett C.A., Barton J.K. Methods to explore cellular uptake of ruthenium complexes. J. Am. Chem. Soc. 2007;129:46–47. doi: 10.1021/ja0677564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Howerton B.S., Heidary D.K., Glazer E.C. Strained ruthenium complexes are potent light-activated anticancer agents. J. Am. Chem. Soc. 2012;134:8324–8327. doi: 10.1021/ja3009677. [DOI] [PubMed] [Google Scholar]
- 57.Hartinger C.G., Jakupec M.A., Zorbas-Seifried S., Groessl M., Egger A., Berger W., Zorbas H., Dyson P.J., Keppler B.K. KP1019, a new redox-active anticancer agent—Preclinical development and results of a clinical phase I study in tumor patients. Chem. Biodivers. 2008;5:2140–2155. doi: 10.1002/cbdv.200890195. [DOI] [PubMed] [Google Scholar]
- 58.Leijen S., Burgers S.A., Baas P., Pluim D., Tibben M., van Werkhoven E., Alessio E., Sava G., Beijnen J.H., Schellens J.H.M. Phase I/II study with ruthenium compound NAMI-A and gemcitabine in patients with non-small cell lung cancer after first line therapy. Investig. New Drugs. 2015;33:201–214. doi: 10.1007/s10637-014-0179-1. [DOI] [PubMed] [Google Scholar]
- 59.Hall J.P., Cook D., Morte S.R., McIntyre P., Buchner K., Beer H., Cardin D.J., Brazier J.A., Winter G., Kelly J.M., et al. X-ray crystal structure of rac-[Ru(phen)2dppz]2+ with d(ATGCAT)2 shows enantiomer orientations and water ordering. J. Am. Chem. Soc. 2013;135:12652–12659. doi: 10.1021/ja403590e. [DOI] [PubMed] [Google Scholar]
- 60.Bhat S.S., Revankar V.K., Khan A., Butcher R.J., Thatipamula K. Supramolecular architecture and photophysical and biological properties of Ruthenium(II) polypyridyl complexes. New J. Chem. 2015;39:3646–3657. doi: 10.1039/C4NJ02394C. [DOI] [Google Scholar]
- 61.Chen L., Peng F., Li G., Jie X., Cai K.R., Cai C., Zhong Y., Zeng H., Li W., Zhang Z., et al. The studies on the cytotoxicity in vitro, cellular uptake, cell cycle arrest and apoptosis-inducing properties of ruthenium methylimidazole complex [Ru(MeIm)4(p-cpip)]2+ J. Inorg. Biochem. 2016;156:64–74. doi: 10.1016/j.jinorgbio.2015.12.016. [DOI] [PubMed] [Google Scholar]
- 62.Mari C., Pierroz V., Rubbiani R., Patra M., Hess J., Spingler B., Oehninger L., Schur J., Ott I., Salassa L., et al. DNA intercalating RuII polypyridyl complexes as effective photosensitizers in photodynamic therapy. Chem. Eur. J. 2014;20:14421–14436. doi: 10.1002/chem.201402796. [DOI] [PubMed] [Google Scholar]
- 63.Kaspler P., Lazic S., Forward S., Arenas Y., Mandel A., Lilge L. A Ruthenium(II) based photosensitizer and transferrin complexes enhance photo-physical properties, cell uptake, and photodynamic therapy safety and efficacy. Photochem. Photobiol. Sci. 2016;15:481–495. doi: 10.1039/C5PP00450K. [DOI] [PubMed] [Google Scholar]
- 64.Fong J., Kasimova K., Arenas Y., Kaspler P., Lazic S., Mandel A., Lilge L. A novel class of ruthenium-based photosensitizers effectively kills in vitro cancer cells and in vivo tumors. Photochem. Photobiol. Sci. 2015;14:2014–2023. doi: 10.1039/C4PP00438H. [DOI] [PubMed] [Google Scholar]
- 65.Padilla R., Rodriguez-Corrales J.A., Donohoe L.E., Winkel B.S.J., Brewer K.J. A new class of Ru(II) polyazine agents with potential for photodynamic therapy. Chem. Commun. 2016;52:2705–2708. doi: 10.1039/C5CC08682E. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Padilla R., Maza W.A., Dominijanni A.J., Winkel B.S.J., Morris A.J., Brewer K.J. Pushing the limits of structurally-diverse light-harvesting Ru(II) metal-organic chromophores for photodynamic therapy. J. Photochem. Photobiol. A. 2016;322:67–75. doi: 10.1016/j.jphotochem.2016.02.006. [DOI] [Google Scholar]
- 67.Caruso F., Monti E., Matthews J., Rossi M., Gariboldi M.B., Pettinari C., Pettinari R., Marchetti F. Synthesis, characterization, and antitumor activity of water-soluble (arene)ruthenium(II) derivatives of 1,3-Dimethyl-4-acylpyrazolon-5-ato ligands. First example of Ru(arene)(ligand) antitumor species involving simultaneous Ru–N7(guanine) bonding and ligand intercalation to DNA. Inorg. Chem. 2014;53:3668–3677. doi: 10.1021/ic403170y. [DOI] [PubMed] [Google Scholar]
- 68.Colina-Vegas L., Villarreal W., Navarro M., de Oliveira C.R., Graminha A.E., Maia P.I.D.S., Deflon V.M., Ferreira A.G., Cominetti M.R., Batista A.A. Cytotoxicity of Ru(II) piano–stool complexes with chloroquine and chelating ligands against breast and lung tumor cells: Interactions with DNA and BSA. J. Inorg. Biochem. 2015;153:150–161. doi: 10.1016/j.jinorgbio.2015.07.016. [DOI] [PubMed] [Google Scholar]
- 69.Liu N., Li X., Huang H., Zhao C., Liao S., Yang C., Liu S., Song W., Lu X., Lan X., et al. Clinically used antirheumatic agent auranofin is a proteasomal deubiquitinase inhibitor and inhibits tumor growth. Oncotarget. 2014;5:5453–5471. doi: 10.18632/oncotarget.2113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zou T., Lum C.T., Lok C.N., Zhang J.J., Che C.M. Chemical biology of anticancer Gold(III) and Gold(I) complexes. Chem. Soc. Rev. 2015;44:8786–8801. doi: 10.1039/C5CS00132C. [DOI] [PubMed] [Google Scholar]
- 71.Nardon C., Fregona D. Gold(III) complexes in the oncological preclinical arena: From aminoderivatives to peptidomimetics. Curr. Top. Med. Chem. 2016;16:360–380. doi: 10.2174/1568026615666150827094500. [DOI] [PubMed] [Google Scholar]
- 72.Rubbiani R., Salassa L., de Almeida A., Casini A., Ott I. Cytotoxic Gold(I) N-heterocyclic carbene complexes with phosphane ligands as potent enzyme inhibitors. ChemMedChem. 2014;9:1205–1210. doi: 10.1002/cmdc.201400056. [DOI] [PubMed] [Google Scholar]
- 73.Holenya P., Can S., Rubbiani R., Alborzinia H., Junger A., Cheng X., Ott I., Wolfl S. Detailed analysis of pro-apoptotic signaling and metabolic adaptation ttriggered by a N-heterocyclic carbene-gold(I) complex. Metallomics. 2014;6:1591–1601. doi: 10.1039/C4MT00075G. [DOI] [PubMed] [Google Scholar]
- 74.Nardon C., Schmitt S.M., Yang H., Zuo J., Fregona D., Dou Q.P. Gold(III)-dithiocarbamato peptidomimetics in the forefront of the targeted anticancer therapy: Preclinical studies against human breast neoplasia. PLoS ONE. 2014;9:e84248. doi: 10.1371/journal.pone.0084248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Akerman K.J., Fagenson A.M., Cyril V., Taylor M., Muller M.T., Akerman M.P., Munro O.Q. Gold(III) macrocycles: Nucleotide-specific unconventional catalytic inhibitors of human topoisomerase I. J. Am. Chem. Soc. 2014;136:5670–5682. doi: 10.1021/ja412350f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Meyer A., Oehninger L., Geldmacher Y., Alborzinia H., Wölfl S., Sheldrick W.S., Ott I. Gold(I) N-heterocyclic carbene complexes with naphthalimide ligands as combined thioredoxin reductase inhibitors and DNA intercalators. ChemMedChem. 2014;9:1794–1800. doi: 10.1002/cmdc.201402049. [DOI] [PubMed] [Google Scholar]
- 77.Liu S., Cao W., Yu L., Zheng W., Li L., Fan C., Chen T. Zinc(II) complexes containing bis-benzimidazole derivatives as a new class of apoptosis inducers that trigger DNA damage-mediated p53 phosphorylation in cancer cells. Dalton Trans. 2013;42:5932–5940. doi: 10.1039/c3dt33077j. [DOI] [PubMed] [Google Scholar]
- 78.Haribabu J., Jeyalakshmi K., Arun Y., Bhuvanesh N.S.P., Perumal P.T., Karvembu R. Synthesis, DNA/protein binding, molecular docking, DNA cleavage and in vitro anticancer activity of Nickel(II) bis(thiosemicarbazone) complexes. RSC Adv. 2015;5:46031–46049. doi: 10.1039/C5RA04498G. [DOI] [Google Scholar]
- 79.Zhang H.R., Liu Y.C., Meng T., Qin Q.P., Tang S.F., Chen Z.F., Zou B.Q., Liu Y.N., Liang H. Cytotoxicity, DNA binding and cell apoptosis induction of a Zinc(II) complex of HBrQ. Med. Chem. Commun. 2015;6:2224–2231. doi: 10.1039/C5MD00406C. [DOI] [Google Scholar]
- 80.Tabrizi L., McArdle P., Erxleben A., Chiniforoshan H. Nickel(II) and Cobalt(II) complexes of lidocaine: Synthesis, structure and comparative in vitro evaluations of biological perspectives. Eur. J. Med. Chem. 2015;103:516–529. doi: 10.1016/j.ejmech.2015.09.018. [DOI] [PubMed] [Google Scholar]
- 81.Wani W.A., Baig U., Shreaz S., Shiekh R.A., Iqbal P.F., Jameel E., Ahmad A., Mohd-Setapar S.H., Mushtaque M., Ting Hun L. Recent advances in iron complexes as potential anticancer agents. New J. Chem. 2016;40:1063–1090. doi: 10.1039/C5NJ01449B. [DOI] [Google Scholar]
- 82.Yu Y., Gutierrez E., Kovacevic Z., Saletta F., Obeidy P., Rahmanto Y.S., Richardson D.R. Iron chelators for the treatment of cancer. Curr. Med. Chem. 2012;19:2689–2702. doi: 10.2174/092986712800609706. [DOI] [PubMed] [Google Scholar]
- 83.Del Oliveira A.C., dalSilva E.G., Rocha D.D., Hillard E.A., Pigeon P., Jaouen G., Rodrigues F.A.R., de Abreu F.C., da Rocha Ferreira F., Goulart M.O.F., et al. Molecular mechanism of action of 2-Ferrocenyl-1,1-diphenylbut-1-ene on HL-60 leukemia cells. ChemMedChem. 2014;9:2580–2586. doi: 10.1002/cmdc.201402219. [DOI] [PubMed] [Google Scholar]