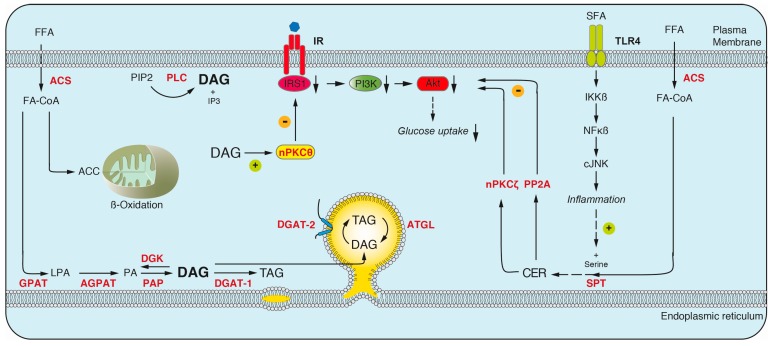
Figure 5.
Lipid intermediates and their interactions with insulin signaling pathways in muscle. Free fatty acids (FFA) are transported into the cell, activated by acyl-CoA-synthase (ACS), and channeled into different pathways: (i) TAG synthesis occurs in four sequential reactions, catalyzed by members of the glycerol-3-phosphate-o-acyltransferase (GPAT), 1-acylglycerol-3-phosphate (AGPAT), phosphatidic acid phosphatase (PAP) and diacylglycerolacyl-transferase (DGAT) enzyme families in the ER and/or LDs. DAG, a key intermediate in TAG synthesis, can alternatively be generated by stimulus-dependent cleavage of phosphatidylinositol 4,5-bisphosphate (PIP2) by phospholipase C (PLC) at the plasma membrane. The lipolysis of TAG by adipose triacylglycerol lipase (ATGL) generates DAG on LDs. Accumulated DAG species recruit and activate nPKCθ which leads to inhibitory phosphorylation of IRS1, downregulation of phosphatidylinositol-kinase 3 (PI3K), AKT and ultimately glucose uptake (indicated by downward arrows); (ii) FA-CoAs are converted to acylcarnitines (ACC) for shuttling and subsequent β-oxidation in mitochondria. Decreased or incomplete oxidation leads to their accumulation and affects insulin sensitivity; (iii) Ceramide synthesis is initiated by serine palmitoyl transferase (SPT) in the ER. Binding of saturated FA (SFA) to toll-like receptor 4 (TLR4) induces an inflammatory response, contributing to increased ceramide synthesis. Ceramides inhibit insulin signaling by decreasing the activity of AKT via protein phosphatase 2A (PP2A) or atypical PKCζ. Lysophosphatidic acid (LPA); Phosphatidic acid (PA); Diacylglycerol kinase (DGK); Inositol-1,4,5-Trisphosphate (IP3).