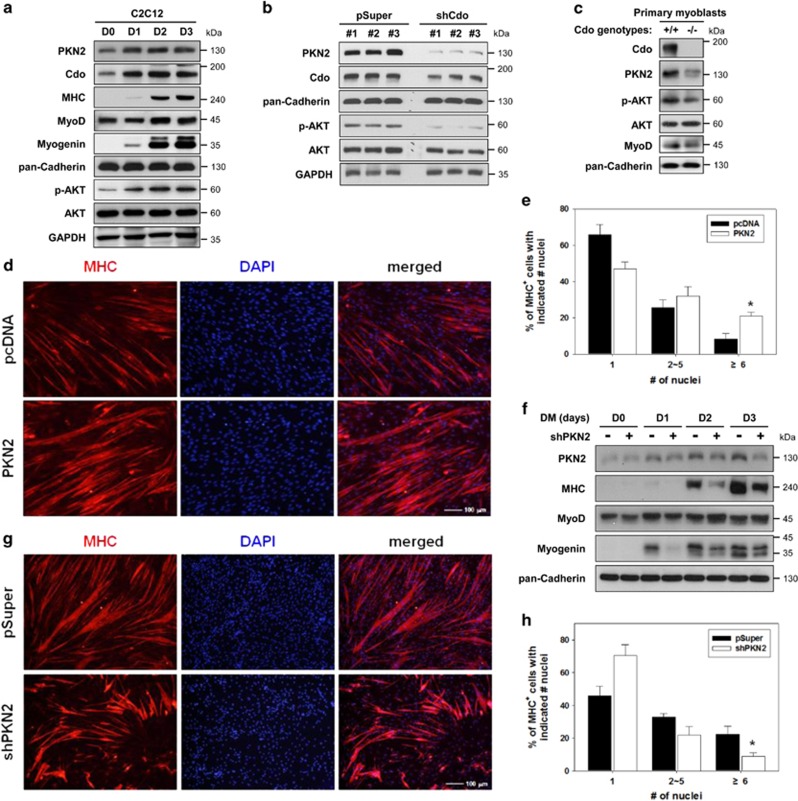
Figure 1.
PKN2 levels are elevated during myoblast differentiation and decreased in Cdo-depleted cells with a concurrent reduction in AKT activation. (a) C2C12 cells were cultured to near confluency (D0) and induced to differentiate in differentiation medium (DM) for total 3 days (D3). Lysates were immunoblotted with antibodies to PKN2, Cdo, MHC, MyoD, myogenin, phosphorylated-AKT (p-AKT) and AKT. GAPDH and pan-Cadherin serve as loading controls. (b) Lysates of C2C12 cells transiently transfected with Cdo or control expression vectors as indicated were immunoblotted with antibodies to PKN2, Cdo, p-AKT and AKT. GAPDH and pan-Cadherin serve as loading controls. (c) Immunoblot analysis for the expression of PKN2, p-AKT and AKT proteins in Cdo+/+ and Cdo−/− primary myoblasts from hindlimb muscles, and pan-Cadherin serves as a loading control. (d) Photomicrographs of C2C12 cells that stably express PKN2 or control vectors were cultured in DM for 2 days, fixed, and immunostained with an antibody to MHC followed by DAPI staining to visualize nuclei. Size bar, 100 μm. (e) Quantification of myotube formation shown in (d). Values represent means of triplicate determinations ±1 S.D. The experiment was repeated three times with similar results. Significant difference from control, *P<0.01. (f) C2C12 cells stably transfected with shPKN2 or control (pSuper) vectors, and cultured to confluency and induced to differentiate for total 3 days. Cell lysates were immunoblotted using antibodies to PKN2, MHC, MyoD, Myogenin and pan-Cadherin as a loading control. (g) Photomicrographs of C2C12 cells stably transfected with PKN2 shRNA or control vectors were cultured in DM for 3 days, fixed and immunostained with an antibody to MHC followed by DAPI staining to visualize nuclei. Size bar, 100 μm. (h) Quantification of myotube formation by cell lines shown in (g). Values represent means of triplicate determinations ±1 S.D. The experiment was repeated three times with similar results. Significant difference from control, *P<0.01