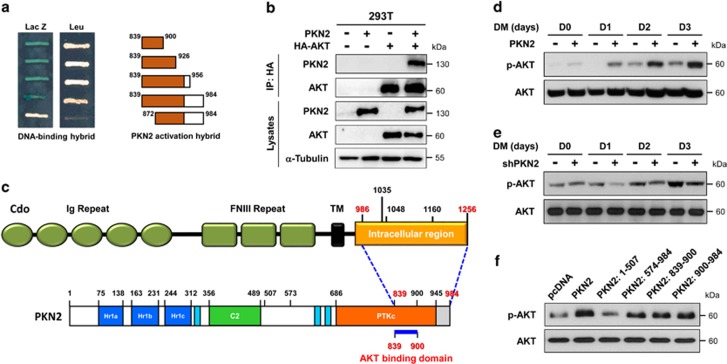
Figure 4.
The C-terminal region of PKN2 is sufficient for the AKT activation. (a) Yeast two-hybrid analysis. The DNA-binding hybrid containing the full-length AKT and the activation hybrids encoding the indicated regions of PKN2 C-terminus were utilized for the interaction ability. The growth on the media lacking leucine and the blue staining for β-galactosidase activity are indicative of the interaction between AKT and PKN2 fragments. Note that the PKN2 lacking AA839-872 failed to interact with AKT. (b) Lysates of 293T cells transfected with PKN2, HA-AKT or control vector were subjected to immunoprecipitation with HA and immunoblotting with PKN2 or HA antibodies. Total lysates were also immunoblotted with antibodies to PKN2 and AKT, and to α-tubulin as a loading control. (c) Schematic diagram of the interacting domains with Cdo and PKN2. (d) C2C12 cells were stably transfected with PKN2 and its deletion mutants or control expression vectors (pcDNA). Lysates of these cell lines were immunoblotted with antibodies to p-AKT and AKT. (e) C2C12 cells were stably transfected with PKN2 overexpression and control vector (pBp), and cultured to confluency and induced to differentiate for total 3 days. Cell lysates were immunoblotted with antibodies to p-AKT and AKT. (f) C2C12 cells stably expressing shPKN2 or control expression vectors (pSuper) were cultured to confluency and induced to differentiate for total 3 days. Cell lysates were immunoblotted with antibodies to p-AKT and AKT