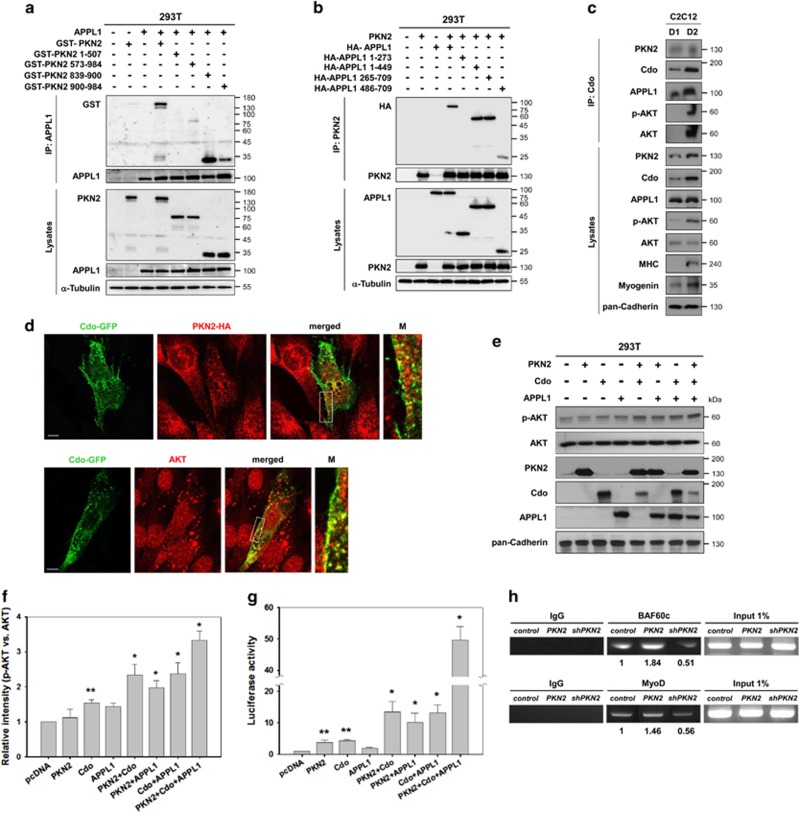
Figure 5.
PKN2, Cdo and APPL1 cooperatively activate AKT and the MyoD-responsive reporter activities. (a) Lysates of 293T transiently transfected with GST-tagged PKN2, its deletion mutants, APPL1 or control expression vectors as indicated were immunoprecipitated with APPL1 antibody and then immunoblotted with antibodies to GST or APPL1. Total lysates were also immunoblotted with antibodies to PKN2 or APPL1, and to α-tubulin as a loading control. (b) Lysates of 293T transiently transfected with HA-tagged APPL1, its deletion mutants, PKN2 or control expression vectors as indicated were immunoprecipitated with PKN2 and then immunoblotted with antibodies to HA or PKN2. Total lysates were also immunoblotted with antibodies to APPL1 or PKN2, and to α-tubulin as a loading control. (c) Lysates of C2C12 cells at D1 and D2 were immunoprecipitated with a Cdo antibody and immunoprecipitates and total cell lysates were assessed by immunoblotting with indicated antibodies. Cadherin expression serves as a loading control. (d) C2C12 cells transfected with Cdo-GFP alone or with HA-PKN2 expression vectors were induced to differentiate for one day and subjected to immunostaining with antibodies to HA (upper panel) or AKT (lower panel). The enlarged images of the boxed areas are shown in the right panels. Size bar, 10 μM. (e) 293T cells were transiently transfected with expression vectors for PKN2, Cdo, APPL1 or combination of these vectors as indicated. The lysates were immunoblotted with antibodies to p-AKT, total AKT, PKN2, Cdo, APPL1 and pan-Cadherin as a loading control. (f) Quantification of three blots similar immunoblots to those shown in (e). The intensity of p-AKT was quantified with the values obtained from control vector-transfected cells set to 1.0. The values represent the means of triplicate determinations±1 S.D. The experiment was repeated three times with similar results. Significant difference from control, *P<0.01, **P<0.05. (g) 10T1/2 cells were co-transfected with a MyoD-responsive luciferase reporter and the expression vectors for MyoD and β-galactosidase as an internal control. In addition, control, PKN2, Cdo and/or APPL1 expression vectors were co-transfected as indicated for 24 h later, the reporter activities were measured and normalized relative to the internal control. The experiment was performed as triplicates and repeated three times with similar results. *P<0.01, **P<0.05. (h) Chromatin immunoprecipitation with anti-MyoD or anti-BAF60c antibodies was performed with C2C12 cells transfected with pSuper, PKN2 or shPKN2. ChIP DNA was assessed by quantitative PCR with primers that specially recognize the MyoD-responsive elements in the Myogenin promoter. All ChIP analysis were performed with three independent chromatin preparations