Abstract
Angiogenesis, the formation of blood vessels from pre-existing ones, is a key event in pathology, including cancer progression, but also in homeostasis and regeneration. As the phenotype of endothelial cells (ECs) is continuously regulated by local biomechanical forces, studying endothelial behaviour in altered gravity might contribute to new insights towards angiogenesis modulation. This study aimed at characterizing EC behaviour after hypergravity exposure (more than 1g), with special focus on cytoskeleton architecture and capillary-like structure formation. Herein, human umbilical vein ECs (HUVECs) were cultured under two-dimensional and three-dimensional conditions at 3g and 10g for 4 and 16 h inside the large diameter centrifuge at the European Space Research and Technology Centre (ESTEC) of the European Space Agency. Although no significant tendency regarding cytoskeleton organization was observed for cells exposed to high g's, a slight loss of the perinuclear localization of β-tubulin was observed for cells exposed to 3g with less pronounced peripheral bodies of actin when compared with 1g control cells. Additionally, hypergravity exposure decreased the assembly of HUVECs into capillary-like structures, with a 10g level significantly reducing their organization capacity. In conclusion, short-term hypergravity seems to affect EC phenotype and their angiogenic potential in a time and g-level-dependent manner.
Keywords: three-dimensional matrigel, altered gravity, capillary-like structures, cytoskeleton, hypergravity pre-stimulation
1. Introduction
Mechanical forces are known to influence the structural and functional properties of tissues at cellular, molecular and genetic levels. Indeed, mechanotransduction induces both rapid responses and slower adaptive changes to a sustained mechanical environment [1]. Endothelial cells (ECs), which constitute the inner layer of blood vessels (the endothelium), are biomechanically responsive cells, given that their phenotype and function are continuously conditioned by local haemodynamics (fluid shear stress, pressure and associated stretch) [2]. Mechanical forces acting on ECs are responsible for regulating angiogenic and inflammatory responses [3,4]. Angiogenesis is the process of blood vessel formation from pre-existing ones, playing a crucial role throughout postnatal life in physiological (e.g. wound healing and the menstrual cycle) and pathological events (e.g. inflammatory diseases and tumour growth), as well as in tissue remodelling and regeneration. Therefore, the ability to modulate the angiogenic response might have strong implications for the development of new strategies in tissue engineering or new approaches in cancer treatment.
Recent advances in the field of biotechnology are generating an increasing interest on ground-based hypergravity (more than 1g) research towards a basic understanding of the effects of accelerations on living systems with sophisticated tools and equipment [5]. Microgravity, on the other hand, is known to accelerate some aspects of cellular senescence, namely via oxidative stress [6], and is considered a model for the study of mechanisms involved in ageing biology [7,8]. Overall, loading forces and gravity have essential effects, yet to be fully understood, on the development and homeostasis of several tissues in the human body [9,10]. Concerning angiogenesis, alterations in gravitational force are known to affect EC integrity and behaviour [8,11,12]. In general, previous studies have demonstrated that both phenotype and function of ECs are affected by changes in gravitational force (i.e. micro- and hypergravity) [11–15]. As reviewed by Maier et al. [8], both micro- and hypergravity have been demonstrated to impact EC migration and proliferation; to increase nitric oxide synthesis, which is an endothelial survival factor with a role in angiogenesis [16]; and to result in cytoskeletal rearrangements. Nevertheless, a lack of conclusive data exist owing to rare test facilities and non-standardized experimental conditions [8] and consequently, EC response to altered gravity conditions, in particular hypergravity, is still poorly understood.
Therefore, the aim of this study was to evaluate (i) the behaviour of ECs immediately after exposure to hypergravity conditions; (ii) the effect of exposing ECs to hypergravity in a three-dimensional microenvironment; and (iii) the behaviour of ECs in a pre-stimulation setting (i.e. ECs cultured at 1g after being exposed to hypergravity). The hypothesis underlying this work is that the pre-stimulation of ECs with hypergravity conditions alters their phenotype and functions in terms of their assembly into capillary-like structures in vitro. For that purpose, cytoskeleton organization was evaluated in two-dimensional cultures, whereas the angiogenic potential was assessed in ECs cultured in three dimensions. Indeed, alterations in the cytoskeleton were evaluated, as this cellular structure is a major responder to mechanical forces [17], including alterations in gravity. Moreover, the functionality of ECs was assessed in a three-dimensional matrigel matrix, which constitutes a suitable environment for EC assembly into capillary-like structures [18]. In addition, different times of exposure (4 and 16 h) and g levels (3 and 10g) were studied.
2. Material and methods
2.1. Culture of human umbilical vein endothelial cells
Human umbilical vein ECs (HUVECs) (ScienCell) were cultured in 0.2% (w/v) gelatin (Merck)-coated flasks in EC growth medium-2 (EGM-2, Lonza) supplemented with 5% (v/v) inactivated fetal bovine serum (FBS, Sigma) and maintained in a humidified atmosphere with 5% CO2 and 95% air at 37°C. The medium was refreshed every 2 days until 90% confluence was reached. Before being used in the experiments, cells were trypsinized through exposure to a trypsin solution for 30 s, followed by incubation at 37°C for 5 min and detachment with the addition of suitable amounts of culture medium, avoiding additional centrifugation steps.
2.2. Hypergravity simulation using a large diameter centrifuge
Hypergravity experiments were performed using the large diameter centrifuge (LDC) (Zeugma) available at the European Space Research and Technology Center (ESTEC, European Space Agency, ESA, Noordwijk, The Netherlands). The LDC has a maximum diameter of 8 m, being composed of four arms that can accommodate a total of six free-swinging gondolas in addition to a central gondola (rotation control), thus supporting hypergravity levels between 1 and 20g [19]. The gondolas swing out, resulting in an acceleration vector perpendicular to the samples surface [20]. In this work, experiments were run either at 3 or 10g during periods of 4 and 16 h, with the LDC needing about 1 min to reach its final rotational speed. For all experiments, cell culture incubators were placed inside the gondolas in order to maintain an atmosphere with 5% CO2 and 95% air at 37°C. Additionally, temperature and CO2 sensors were included in the set-up.
2.3. Exposure of human umbilical vein endothelial cells to hypergravity under two- or three-dimensional conditions
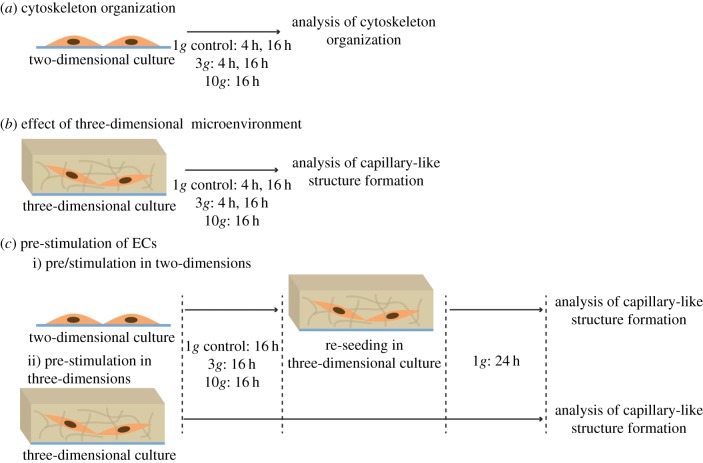
To evaluate the influence of hypergravity on the morphology and organization of ECs, HUVECs were cultured either in two or three dimensions, exposed to hypergravity (4 h at 3g and 16 h at 3 and 10g) and immediately analysed or further cultured in three dimensions for 24 h under normogravity (1g) conditions and then analysed. Figure 1 schematically describes the experimental procedure.
Figure 1.
Schematic of the experimental design. (a) HUVECs were exposed to hypergravity in two-dimensional cultures and then modifications in cytoskeleton organization were analysed. (b) HUVECs were exposed to hypergravity in three-dimensional cultures, and then the number of capillary-like structures was determined. (c) HUVECs were pre-stimulated under hypergravity conditions and then exposed to normogravity (1g) for 24 h, as follows: (i) HUVECs were exposed to hypergravity in two-dimensional cultures, re-seeded in three-dimensional cultures and re-incubated for an additional period of 24 h at 1g; subsequently, the number of capillary-like structures was determined; (ii) HUVECs were exposed to hypergravity in three-dimensional cultures (in condition b) and subsequently re-incubated for an additional period of 24 h at 1g and the number of capillary-like structures was again quantified. (Online version in colour.)
For two-dimensional cultures, cells were seeded on top of 0.2% (w/v) gelatin-coated 13 mm tissue culture polystyrene coverslips inside 24-well plates. HUVECs were seeded at a density of 3.5 × 104 cells well−1 in 500 μl of EGM-2 and allowed to adhere for 1 h 30 at 1g at 37°C in a humidified 5% CO2 atmosphere. Then, plates were transferred to the LDC to be exposed to hypergravity conditions. After each LDC run, part of the cells cultured under two-dimensional conditions were fixed for analysis, whereas the remaining cells of the two-dimensional cultures were transferred to three-dimensional normogravity cultures before analysis.
For three-dimensional cultures, an overlay method was used. For this, growth factor reduced basement membrane matrix (GFR-Matrigel, 200 μl well−1; Corning) was added to each well in 24-well culture plates and incubated at 37°C for 30 min to allow for matrigel polymerization. Then, 3 × 104 cells well−1 in 500 μl of EGM-2 were added on top of the matrigel layer. HUVECs were maintained at 1g for 1 h 30 at 37°C in a humidified 5% CO2 atmosphere to allow their adhesion to matrigel. Afterwards, plates were transferred to the LDC to be exposed to hypergravity conditions, as previously mentioned. The assembly of HUVECs into capillary-like structures was evaluated as described below.
2.4. Characterization of cell behaviour
2.4.1. Cytoskeleton organization by immunofluorescence and cell morphology analysis
Immediately after hypergravity exposure, part of the two-dimensional samples were fixed in 4% (w/v) paraformaldehyde (Sigma). Then, cells were permeabilized with 0.2% (v/v) Triton-X 100 (Merck) for 10 min, washed with PBS, blocked with 1% (w/v) bovine serum albumin (Sigma) in PBS and incubated with mouse anti-human β-tubulin (Sigma, T4026, 1 : 1000) primary antibody for 1 h at room temperature. Subsequently, cells were washed with PBS and incubated with Alexafluor 555 donkey anti-mouse (TermoFisher Scientific, A-31579, 1 : 1000) for 1 h at room temperature. All antibodies were diluted in 1% (w/v) bovine serum albumin in PBS. F-actin was stained by incubating cells with phalloidin (fluorescein isothiocyanate labelled phalloidin from Amanita phalloides, Sigma, 1 : 200) for 20 min. Cell nuclei were counterstained with 4,6-diamidino-2-phenyindole, dilactate (DAPI, 5 μg µl−1, Sigma, 1 : 1000 in PBS) for 10 min. All samples were visualized and photographed under a confocal microscope (CLSM Leica SP2 AOBS; Leica Microsystems).
Cell morphology was then evaluated in a minimum of 50 cells per condition using ImageJ software (http://rsb.info.nih.gov/ij). Cells were evaluated in terms of area, circularity, solidity and roundness as described elsewhere [21]: (i) circularity = 4π area perimeter−2, where area and perimeter are the area and perimeter of each cell; (ii) solidity = area/convex area, where convex area is the area of the smallest convex polygon containing the cell; and (iii) roundness = width length−1 of the smallest rectangle enclosing the cell.
In addition, the overall actin filament orientation was studied using two-dimensional Fourier transform and principal component analysis (PCA) [22]. First, the Fourier transform was used to retrieve the frequency information of the green channel of the confocal microscopy images. Then, PCA was used to retrieve the main components of the frequency's magnitude. The ratio between the eigenvalues of the two PCs enabled assessment of the overall orientation of the filaments. A higher ratio value is indicative of a higher preferred orientation.
2.4.2. Assembly into capillary-like structures by three-dimensional matrigel assay
In order to evaluate the influence of hypergravity on the ability of HUVECs to organize into capillary-like structures, a three-dimensional matrigel assay was performed. For this, cells were seeded on top of a thick layer of matrigel, as described in detail above. Matrigel samples were observed and photographed using an inverted light microscope at two time points: (i) immediately after leaving the LDC and (ii) 24 h after 1g culture following hypergravity pre-stimulation. In the pre-stimulation condition, HUVECs were first exposed to hypergravity in two- or three-dimensional cultures. After being cultured in two dimensions under hypergravity conditions, HUVECs were trypsinized and seeded onto matrigel (figure 1c). In the case of three-dimensional cultures, the number of capillary-like structures was determined after exposure to hypergravity, and then these cultures were re-incubated for an additional 24 h period at 1g (figure 1c). Subsequently, images were collected and the number of capillary-like structures was quantified. For each condition, images from the middle and the corners of three independent wells were acquired. The number of capillary-like structures formed by ECs were manually quantified using the open-sourced ImageJ software (v. 1.42).
2.5. Statistical analyses
All experiments were performed in triplicate. Quantifications are expressed as mean ± standard deviation. One-way analysis of variance with Tukey tests was used to compare between more than two groups. A difference between experimental groups was considered significant with a confidence interval of 95%, whenever p < 0.05.
3. Results
3.1. Adaptive response of human umbilical vein endothelial cells under hypergravity
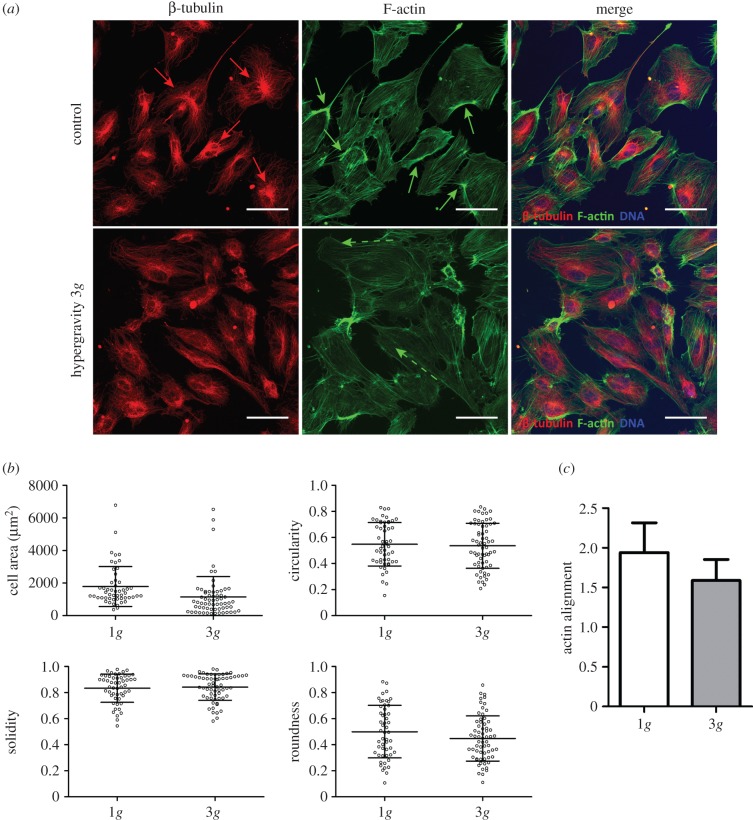
To understand the acute impact of acceleration on the behaviour of ECs, HUVECs were cultured under 3g hypergravity for 4 h. After the LDC run, the characteristic polygonal shape of ECs was still maintained in two-dimensional cultures (figure 2a), with no significant differences being observed in terms of cell area, circularity, solidity or roundness (figure 2b). Nevertheless, slight differences were detected in the organization of cytoskeletal proteins. Indeed, β-tubulin was found to localize mainly around the nuclei in the control cells (figure 2a, red arrows), which was not observed in cells cultured under 3g hypergravity condition. Additionally, the dense peripheral bodies of actin, which were easily visualized in control HUVECs (figure 2a, green arrows), were found to be less pronounced in HUVECs subjected to 3g during a 4 h period and to align with the major axis of the cell (figure 2a, dashed green arrows), but with no overall preferential orientation (figure 2c).
Figure 2.
Microtubular and actin cytoskeletal organization in HUVECs cultured at 3g for 4 h. (a) Confocal images of the cytoskeleton of HUVECs cultured at 1g (top panel) and at 3g (bottom panel) for 4 h. β-tubulin (red), F-actin (green) and DAPI (blue). Scale bars, 50 µm. (b) Quantitative evaluation of cell morphology: area, circularity, solidity and roundness. (c) Overall actin filament orientation.
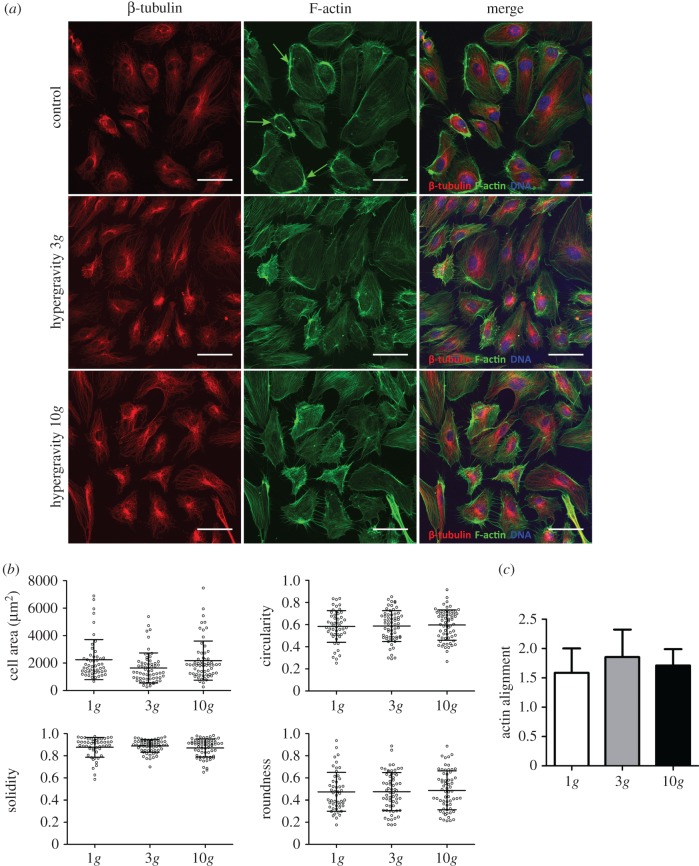
Given that no significant effects were observed on HUVECs exposed to 3g for 4 h, the behaviour of ECs cultured in two dimensions was studied during a longer exposure time (16 h) and a higher g-level (10g). Similar to the response observed at 3g for 4 h, HUVECs cultured either at 3 or 10g for 16 h exhibited a characteristic polygonal shape (figure 3a), and no major differences in the evaluated parameters regarding cell morphology were notable (figure 3b). Moreover, the dense peripheral bodies of actin were clearly detected in HUVECs from control conditions (figure 3a, green arrows), although less pronounced in cells exposed to hypergravity and with no overall orientation (figure 3c). Additionally, no differences were evident regarding β-tubulin expression or microtubule organization in HUVECs. Here, a radial distribution was observed inside cells, with a less evident localization around cell nuclei in all conditions tested (figure 3a).
Figure 3.
Microtubular and actin cytoskeletal organization in HUVECs cultured at 3 and 10g for 16 h. (a) Confocal images of the cytoskeleton of HUVECs cultured at 1g (top panel), 3g (middle panel) and 10g (bottom panel) for 16 h. β-tubulin (red), F-actin (green) and DAPI (blue). Scale bars, 50 µm. (b) Quantitative evaluation of cell morphology: area, circularity, solidity and roundness. (c) Overall actin filament orientation.
3.2. Effect of three-dimensional microenvironment
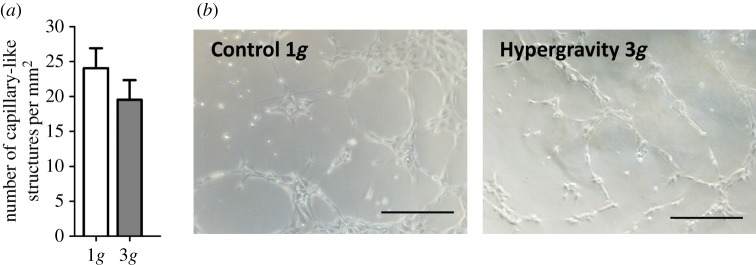
To better understand the effect of hypergravity on the in vitro functionality of HUVECs, a three-dimensional matrigel assay was performed. Herein, the ability of ECs to assemble into a three-dimensional capillary-like network when cultured in a three-dimensional matrix was evaluated under the same hypergravity conditions previously tested in two dimensions. After 4 h of exposure to 3g hypergravity, although there was a slight reduction (not statistically significant) in the formation of capillary-like structures compared with control HUVECs (figure 4a,b), there was no significant inhibition of the assembly capacity of HUVECs in matrigel. Indeed, this level and time of exposure to hypergravity was not sufficient to affect the characteristic assembly capability of HUVECs. Therefore, HUVECs cultured in three dimensions were also tested under a longer exposure time (16 h) and at a higher g-level (10g), as described for two-dimensional cultures. Here, a decrease in the number of capillary-like structures formed in the three-dimensional matrigel assay was observed for HUVECs cultured both at 3 and 10g for 16 h (figure 5a,b). Moreover, the exposure of HUVECs to a 10g hypergravity level significantly reduced their ability to organize when embedded in the matrigel matrix, as determined by the number of capillary-like structures (figure 5a) and observed in optical microscopic images (figure 5b).
Figure 4.
Capillary-like structure formation by HUVECs cultured in three dimensions at 3g for 4 h. (a) Average number of capillary-like structures per mm2 formed by HUVECs after 4 h cultured under hypergravity (n = 5, p < 0.05); (b) Representative microscope images of capillary-like structures formed by HUVECs. Scale bars, 100 µm. (Online version in colour.)
Figure 5.
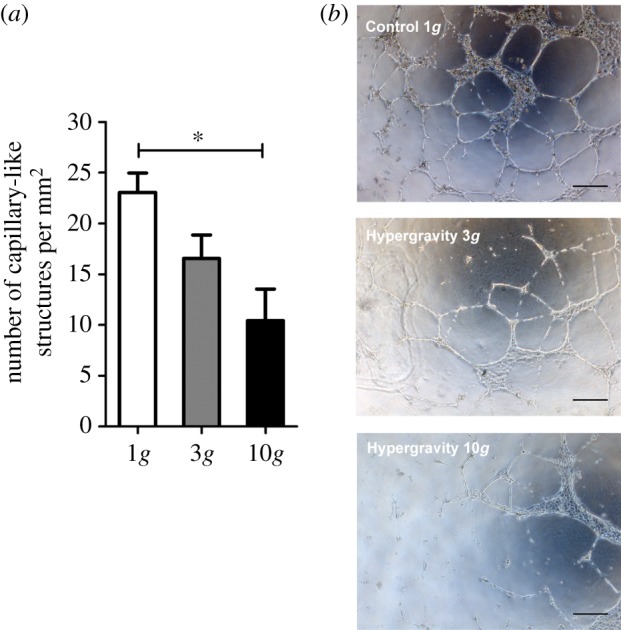
Capillary-like structure formation by HUVECs cultured in three-dimension at 3 and 10g for 16 h. (a) Average number of capillary-like structures per mm2 formed by HUVECs after 16 h cultured under hypergravity. Asterisk denotes statistically significant differences (n = 5, p < 0.05); (b) Representative microscope images of capillary-like structures formed by HUVECs. Scale bars, 100 µm. (Online version in colour.)
3.3. Pre-stimulation of human umbilical vein endothelial cells under hypergravity
To better understand whether the hypergravity-induced reduction in the formation of capillary-like structures could be reversed upon return to 1g, HUVECs were cultured in the three-dimensional matrigel assay at normal gravity conditions for an additional period of 24 h after hypergravity exposure. For this, HUVECs were first exposed to hypergravity in two- or three-dimensional cultures. HUVECs cultured in two dimensions under hypergravity conditions at 3 or 10g for 16 h were then trypsinized and seeded on top of a matrigel layer after determining their viability as described in §3.1. These cells were cultured in three dimensions for 24 h at 1g. By culturing collected HUVECs for an additional period of 24 h in three-dimensional matrigel, no differences were observed in terms of the ability of these cells to organize into capillary-like structures (figure 6a,b). Here, similar values were observed for both hypergravity conditions (16 ± 3 and 17 ± 2 capillary-like structures mm−2 for 3 and 10g, respectively) and the control at 1g (17±2 capillary-like structures mm−2).
Figure 6.
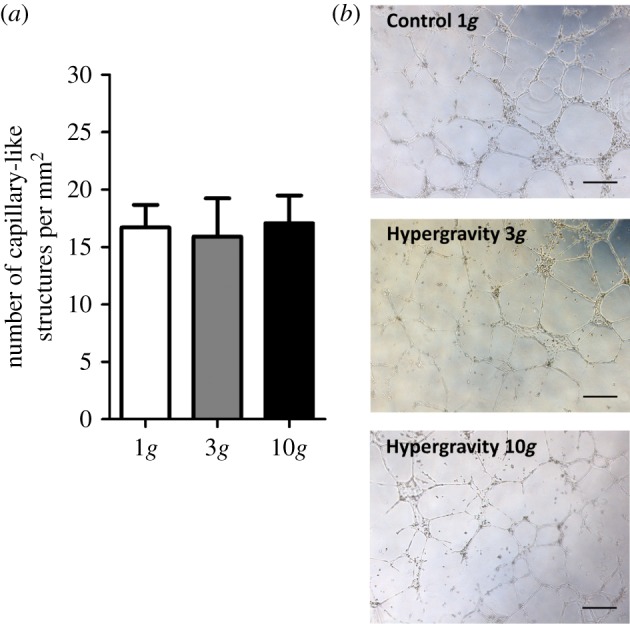
Capillary-like structure formation by HUVECs pre-stimulated in two-dimensional culture at 3g and 10g for 16 h. (a) Average number of capillary-like structures per mm2 formed by HUVECs after 16 h of hypergravity pre-stimulation in two dimensions and seeding in three-dimensional matrigel for 24 h at 1g. Asterisk denotes statistically significant differences (n = 5, p < 0.05); (b) representative microscope images of capillary-like structures formed by HUVECs. Scale bars, 100 µm. (Online version in colour.)
Additionally, HUVECs cultured in three-dimensional matrigel under hypergravity conditions for 16 h were also maintained in culture for an additional period of 24 h at 1g. In this case, the number of capillary-like structures was still significantly lower in the 10g compared to the 1g condition (figure 7a,b), as it was immediately after hypergravity exposure (figure 5). Therefore, the effect of hypergravity on capillary-like structure formation could not be reversed by continued culturing within 24 h under 1g. In addition, there was an overall decrease in the number of capillary-like structures after the additional 24 h period at 1g for all three gravity levels analysed. Indeed, in control cells, there was a reduction from 23 ± 2 to 17 ± 2 capillary-like structures mm−2. A similar trend was observed in HUVECs exposed to a pre-stimulation at 3 and 10g, with the number of capillary-like structures mm−2 decreasing from 16 ± 2 (after 16 h of hypergravity exposure at 3g) to 13 ± 2 (after the 24 h additional period) and from 10 ± 3 (after 16 h of hypergravity exposure at 10g) to 9 ± 3 (after the 24 h additional period), suggesting the disintegration of the pre-formed capillary-like structures.
Figure 7.
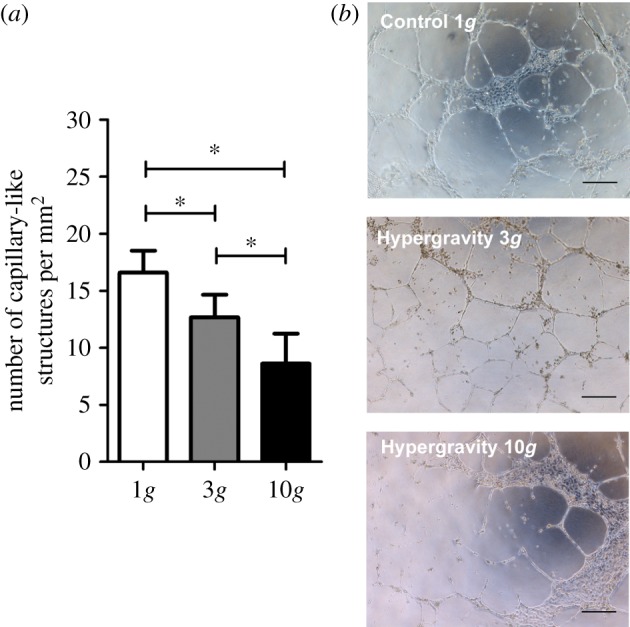
Pre-stimulation of HUVECs cultured in three-dimensional matrigel at 3g and 10g for 16 h. (a) Average number of capillary-like structures per mm2 formed by HUVECs after 16 h of hypergravity pre-stimulation and 24 h at 1g. Asterisk denotes statistically significant differences (p < 0.05); (b) representative microscope images of capillary-like structures formed by HUVECs. Scale bars, 100 µm. (Online version in colour.)
4. Discussion
There is still little information concerning the behaviour of ECs and angiogenesis under hypergravity, with a few published experimental results even being contradictory, as reviewed by Maier et al. [8]. In this work, the influence of hypergravity on EC behaviour was assessed both in two- and in three-dimensional cultures of HUVECs. Herein, 3g hypergravity was selected given that EC behaviour, and in particular HUVEC behaviour, has already been studied under 3g [23]. Moreover, in order to investigate the behaviour of ECs, including their ability to organize into capillary-like structures, when exposed to higher g forces, 10g hypergravity was chosen, based on the fact that other tissue culture experiments have been previously performed at 10g using the LDC system [24], allowing a comparison of the obtained results with existing literature. Therefore, this experiment aimed at investigating the influence of hypergravity on EC behaviour with a special focus on angiogenesis, particularly on cytoskeletal organization and capillary-like structure formation using different culture conditions (two and three dimensions), time exposure periods (4 and 16 h) and g levels (3 and 10g).
4.1. Endothelial cell morphology and cytoskeleton organization under hypergravity
Previous studies on HUVECs did not address the direct effects of hypergravity on cell viability, whereas studies on neuron-like cells and osteoblasts have reported no significant influence on cell viability after exposure to hypergravity [25,26]. In respect to ECs, a noteworthy aspect is that EC behaviour under altered gravity (both micro- and hypergravity) seems to be highly dependent on the source of ECs, with ECs derived from micro- and macrovessels responding differently to changes in gravitational forces [8]. Strikingly, at the gene level, a hypergravity-induced reduction of the expression of transcription genes encoding for pro-apoptotic factors (e.g. Fas, FasL and Bcl-XL) and an upregulated expression of the anti-apoptotic factor NFκB was reported for ECs both from micro- and macrovascular origin [11,12]. Although not directly associated with cell viability, these results indicate maintenance of EC survival under hypergravity conditions. Moreover, in this work, the effect of hypergravity in EC morphology was assessed as previously described [21]. Although hypergravity has been suggested to affect cell morphology, particularly for mesenchymal stem cells cultured at 20g for 3 h [21], no major alterations were observed in this work. These results suggest that morphological adaptation upon hypergravity exposure may be dependent on distinct parameters, including cell type and g-level. Similarly, previous results have shown no significant alterations in actin alignment or structure in HUVECs exposed to 8g for increasing periods of time (5–30 min), in comparison with normogravity conditions [27]. Additionally, as mentioned above, studies regarding the influence of hypergravity on EC behaviour are contradictory owing to the very distinct conditions tested (e.g. cell origin and g-level or exposure duration). Nonetheless, an agreement exists regarding a general effect of hypergravity on cytoskeletal rearrangements [8]. The cytoskeleton is a key player in orchestrating cellular adaptation to mechanical stress, including alterations of gravity [17,28]. In our study, slight changes could be observed in microtubular and actin cytoskeletal organization and distribution. Although not very substantial, small changes in cytoskeletal structure are known to have major effects on gene expression [17]. Other studies reported more vigorous effects on cytoskeletal structure [12,29]. Differences between the studies could be due to the shorter duration of experiments, levels of hypergravity used, as well as differences in EC origin, compared with our study. In this work, typical dense peripheral bodies of actin were found to be less pronounced after a shorter exposure (4 h) to 3g hypergravity level. Morbidelli et al. reported a similar effect on actin organization, with control bovine aortic ECs (BAECs) exhibiting a typical peripheral ring, which faded in BAECs exposed to discontinuous hypergravity exposure (5 × 10 min at 10g with 10 min intervals at 1g) that presented transcytoplasmic stress fibres [12]. On the contrary, a previous study with HUVECs reported an enforcement of the cytoskeleton, with F-actin being organized in a densely packed peripheral ring and its content being significantly increased after exposure to hypergravity (2g for 15 min) [29]. The authors reported an active adaptation and a fast remodelling of EC behaviour through alterations in cell–cell and cell–matrix adhesions, upon very short-term exposure to hypergravity (2g, 15 min) [29].
With regard to microtubular organization, the shorter exposure tested in this work resulted in a radial distribution of β-tubulin in HUVECs cultured under 3g hypergravity for 4 h, in comparison with control cells, which exhibited this protein mainly located around the nuclei. A similar radial distribution was observed in HUVECs cultured under 3 and 10g hypergravity levels for 16 h. These data are in contrast to the existing literature. Morbidelli et al. found that microtubules of hypergravity-exposed BAECs tended to gather mainly in the perinuclear area, in comparison with a homogeneous distribution of tubulin in control cells [12]. Given the long-term exposure we studied (4 and 16 h), the lack of a pronounced change in cytoskeleton might result from an adaptive response of cytoskeletal proteins orchestrated by ECs over time. Results regarding hypergravity effects on cytoskeleton rearrangements, particularly at the molecular level, are still scarce. However, previous studies in microgravity conditions evidenced a rearrangement of F-actin after an initial short-term depolimerization response of stress fibres [30]. Here, an initial disruption of F-actin in HUVECs was observed after 5 min, with this effect being more pronounced after 1 h of exposure to simulated microgravity; whereas longer exposure periods (24 h) resulted in a re-organization of F-actin, with a gradual recovery towards a stable state [30]. Thus, HUVECs dynamically change their cytoskeleton as a major responder to altered gravity, with fast changes being detected early after exposure to altered mechanical conditions and subsequent long-term adaptations occurring after prolonged exposure to this stimulus. A similar response may be occurring under the hypergravity conditions tested in this study, as no well-defined differences were observed in F-actin organization between hypergravity-stimulated cells and the control at 1g. Overall, currently available research on the impact of hypergravity on ECs is contradictory and, therefore, additional studies at the single-cell, molecular level under fixed experimental settings are necessary towards generating new insights into the influence of hypergravity on cytoskeleton dynamics.
4.2. In vitro angiogenesis under hypergravity
In order to further evaluate the influence of hypergravity on the behaviour of HUVECs, the functionality of these cells was assessed by determining their ability to organize into capillary-like structures in vitro. Over the years, different assays have been developed to assess the angiogenic potential of ECs [18,31,32], with the three-dimensional matrigel assay being the most commonly used technique. Hence, besides being cultured in two dimensions, HUVECs were also exposed to hypergravity in three-dimensional culture using a matrigel matrix. Overall, exposure to hypergravity resulted in a decreased ability of HUVECs to organize into capillary-like structures. Indeed, exposure to 3g hypergravity for 4 h already resulted in a slight reduction (although not significant) of the number of capillary-like structures formed, in comparison to the 1g control. The same tendency was observed for a longer exposure period, with HUVECs exhibiting a diminished ability to organize into capillary-like structures after being cultured at 3g for 16 h. Accordingly, Spinsi et al. reported inhibition of the angiogenic behaviour of HUVECs cultured in a three-dimensional collagen gel and exposed to 3g hypergravity for 48 h [33]. Despite the fact that no quantification is presented by the authors, results from this work showed that the formation of capillary-like structures was strongly impaired after culturing cells in a three-dimensional collagen gel at 3g with the addition of basic fibroblast growth factor [33]. In this study, although the number of capillary-like structures formed by HUVECs in three-dimensional matrigel is reduced, no significant differences were detected between cells exposed to 3g for 4 or 16 h and the control at 1g. These results suggest that matrigel might be a more permissive model than collagen gels in terms of EC organization under hypergravity conditions. Accordingly, Spinsi et al. also stated a diminished cell migration into the collagen matrix under hypergravity [33]. In fact, collagen cross-linking generates a tighter packed three-dimensional matrix, which may delay the passage of cells through the gel network, whereas matrigel produces thick but loosely cross-linked gels and it has a great amount of growth factors in its composition [34]. Indeed, stiffness of a three-dimensional matrix has been demonstrated to result in altered cell behaviour, namely of cultured dermal fibroblasts [35] and mesenchymal stem cells [36] not only in terms of cell morphology, but also regarding gene expression potentially impacting the function of cells. Herein, matrigel provides not only a physical support for EC organization, but also an array of biochemical signals that stimulate angiogenesis. Additionally, longer exposure times might not be sufficient to diminish the formation of capillary-like structures, as no significant differences were observed, after culturing HUVECs at 3g for 4 and 16 h, respectively. However, when increasing the g-force applied, from 3 to 10g, a significant reduction was observed in the assembly capacity of HUVECs, when compared with the control. These results suggest that the decrease in the angiogenic behaviour of HUVECs in the three-dimensional matrigel assay might be more dependent on the g-force than on the time of exposure to hypergravity. However, additional studies should be performed in order to clarify the influence of acceleration on the functional behaviour of living cells, namely ECs and angiogenesis. Results concerning the effects of mechanical stress on the assembly of ECs into capillary-like structures in vitro are still poorly understood. For instance, simulated microgravity conditions have been reported to enhance capillary-like structure formation by HUVECs cultured in matrigel [37]. Nonetheless, considering microgravity and hypergravity as having opposite effects is not a straightforward conclusion [8].
Therefore, to investigate if the diminished ability of HUVECs to organize into capillary-like structures under hypergravity could be reversed upon return to 1g, then a pre-stimulation study was performed. For this purpose, HUVECs were first exposed to hypergravity (3 and 10g for 16 h) either in two- or three-dimensional cultures. Here, cells cultured in two dimensions were trypsinized after hypergravity stimulation and seeded in a three-dimensional matrigel layer, being incubated for an additional period of 24 h at 1g. In three-dimensional cultures, cells were analysed immediately upon hypergravity stimulation as described above and re-incubated at 1g for 24 h. For both cases, the number of capillary-like structures was determined after the incubation period under normal gravity conditions. Pre-stimulating HUVECs in two dimensions for 16 h (both at 3 and 10g) and subsequently culturing them in three-dimensional matrigel showed no impact on their ability to organize into capillary-like structures, showing that centrifugation and a subsequent trypsinization procedure of ECs prior to cell seeding on three-dimensional matrigel does not impact their angiogenic behaviour.
However, HUVECs subjected to pre-stimulation in three-dimensional matrigel could not recover from the reduction in their assembly capacity. Here, cells were first exposed to hypergravity in three-dimensional culture in matrigel for 16 h; after, the number of capillary-like structures formed was determined, and cells were further incubated in three-dimensional matrigel for an additional 24 h period at 1g.
To the best of our knowledge, we are the first to study the effect of pre-stimulating ECs under hypergravity and further evaluating their functional behaviour in a three-dimensional microenvironment. As previously reported, ECs alone are able to organize in matrigel; however, the number of capillary-like structures formed starts to decrease over time [38]. Although matrigel provides a three-dimensional microenvironment to support EC assembly, in an in vivo scenario, vascular structures need to be stabilized by the presence of other supporting cells, such as mural cells (e.g. smooth muscle cells, pericytes) and fibroblasts [39]. For instance, dermal fibroblasts have been demonstrated to support the organization of ECs into capillary-like structures, providing essential matrix components and maintaining these structures for at least 21 days in culture [38,40], showing the need to have other cells present in the system to promote stabilization of capillary-like networks. Hence, future studies using more complex systems with co-cultured cells might be helpful in order to highlight stronger evidence on the angiogenic behaviour under hypergravity in an in vivo-like microenvironment. Moreover, the use of simulated hypergravity conditions in the field of tissue engineering is still relatively unexplored and the modulation of cellular functions using such stimulation hold a strong potential towards the development of novel strategies.
Acknowledgements
The experiments reported here were performed in the framework of the Spin Your Thesis! 2014 programme, organized by ESA Education Office. The authors are grateful to staff of ESA Education Office, Natacha Callens and Lily Ha, for support, and to Alan Dowson for the technical support before and during the campaign. R.C.-A. acknowledges the PhD grant SFRH/BD/96593/2013 from FCT-Fundação para a Ciência e a Tecnologia.
5. Conclusion
This study showed that although hypergravity conditions alter EC behaviour, cell viability seems to be unaffected. Changes in the expression and organization of the cytoskeletal proteins β-tubulin and F-actin after exposing HUVECs to 3 and 10g for 4 and 16 h could be observed. Although not very obvious, these results suggest the existence of an adaptive response orchestrated by ECs cultured under hypergravity conditions. Regarding angiogenesis, hypergravity leads to a decrease in capillary-like structure formation by HUVECs cultured in three-dimensional matrigel. This effect is strongly dependent on the g-level. Altogether, this understanding may generate knowledge towards the establishment of an emerging field with potential applications in cell-based therapies for tissue engineering strategies through the modulation of angiogenesis.
Authors' contributions
R.C.-A., D.T.O.C., M.J.S.F., G.A. designed the study, carried out laboratory work, participated in data collection and statistical analysis; R.C.-A. wrote the manuscript; M.E.G. helped draft the manuscript; J.J.W.A.v.L., K.V.d.H. and P.L.G. helped design the study, coordinated the study and helped draft the manuscript. All authors gave final approval for publication.
Competing interests
We have no competing interests.
Funding
This work was supported by the Spin Your Thesis! 2014 programme, organized by ESA Education Office. Additional funding include the European Regional Development Fund (ERDF) through the Programa Operacional Factores de Competitividade – COMPETE, and by Portuguese funds through FCT-Fundação para a Ciência e a Tecnologia in the framework of FCT-POPH-FSE, the research grant PEst-C/SAU/LA0002/2011, and co-financed by the North Portugal Regional Operational Programme (ON.2-O Novo Norte) in the framework of the project NORTE-01-0145-FEDER-000012, under the National Strategic Reference Framework (NSRF).
References
- 1.Davies PF, Tripathi SC. 1993. Mechanical stress mechanisms and the cell. An endothelial paradigm. Circ. Res. 72, 239–245. ( 10.1161/01.RES.72.2.239) [DOI] [PubMed] [Google Scholar]
- 2.Davies PF. 2009. Hemodynamic shear stress and the endothelium in cardiovascular pathophysiology. Nat. Rev. Cardiol. 6, 16–26. ( 10.1038/ncpcardio1397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bryan MT, Duckles H, Feng S, Hsiao ST, Kim HR, Serbanovic-Canic J, Evans PC. 2014. Mechanoresponsive networks controlling vascular inflammation. Arteriosclerosis, Thromb. Vasc. Biol. 34, 2199–2205. ( 10.1161/ATVBAHA.114.303424) [DOI] [PubMed] [Google Scholar]
- 4.Li S, Huang NF, Hsu S. 2005. Mechanotransduction in endothelial cell migration. J. Cell. Biochem. 96, 1110–1126. ( 10.1002/jcb.20614) [DOI] [PubMed] [Google Scholar]
- 5.van Loon JJWA. 2001. Hypergravity studies in The Netherlands. J. Gravit. Physiol. 8, P-139–P-142. [PubMed] [Google Scholar]
- 6.Wang J, et al. 2009. Simulated microgravity promotes cellular senescence via oxidant stress in rat PC12 cells. Neurochem. Int. 55, 710–716. ( 10.1016/j.neuint.2009.07.002) [DOI] [PubMed] [Google Scholar]
- 7.Biolo G, Heer M, Narici M, Strollo F. 2003. Microgravity as a model of ageing. Curr. Opin Clin. Nutr. Metab. Care 6, 31–40. ( 10.1097/00075197-200301000-00006) [DOI] [PubMed] [Google Scholar]
- 8.Maier JAM, Cialdai F, Monici M, Morbidelli L. 2015. The impact of microgravity and hypergravity on endothelial cells. Biomed. Res. Int. 2015, 434803 ( 10.1155/2015/434803) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Genchi GC, Rocca A, Marino A, Grillone A, Mattoli V, Ciofani G. 2016. Hypergravity as a tool for cell stimulation: implications in biomedicine. Front. Astron. Space Sci. 3 ( 10.3389/fspas.2016.00026) [DOI] [Google Scholar]
- 10.Mammoto T, Ingber DE. 2010. Mechanical control of tissue and organ development. Development 137, 1407–1420. ( 10.1242/dev.024166) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Monici M, Marziliano N, Basile V, Romano G, Conti A, Pezzatini S, Morbidelli L. 2006. Hypergravity affects morphology and function in microvascular endothelial cells. Microgravity-Sci. Technol. 18, 234–238. ( 10.1007/BF02870417) [DOI] [Google Scholar]
- 12.Morbidelli L, Marziliano N, Basile V, Pezzatini S, Romano G, Conti A, Monici M. 2009. Effect of hypergravity on endothelial cell function and gene expression. Microgrvaity-Sci. Technol. 21, 135–140. ( 10.1007/s12217-008-9067-7) [DOI] [Google Scholar]
- 13.Carlsson S, Bertilaccio M, Ballabio E, Maier J. 2003. Endothelial stress by gravitational unloading: effects on cell growth and cytoskeletal organization. Biochim. Biophys. Acta 1642, 173–179. ( 10.1016/j.bbamcr.2003.08.003) [DOI] [PubMed] [Google Scholar]
- 14.Grenon S, Jeanne M, Aguado-Zuniga J, Conte M, Hughes-Fulford M. 2013. Unloading in endothelial cells: association between caveolins, inflammation and adhesion molecules. Sci. Rep. 3, 1494 ( 10.1038/srep01494) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mariotti M, Maier JAM. 2009. Human micro- and macrovascular endothelial cells exposed to simulated microgravity upregulate hsp70. Microgrvaity-Sci. Technol. 21, 141–144. ( 10.1007/s12217-008-9066-8) [DOI] [Google Scholar]
- 16.Cooke JP, Losordo DW. 2002. Nitric oxide and angiogenesis. Circulation 105, 2133–2135. ( 10.1161/01.CIR.0000014928.45119.73) [DOI] [PubMed] [Google Scholar]
- 17.Hughes-Fulford M, Boonstra J. 2010. Cell mechanotransduction: cytoskeleton and related signalling pathways. In Cell mechanochemistry. Biological systems and factors inducing mechanical stress, such as light, pressure and gravity (eds Monici M, Loon JWAV), pp. 75–95. Trivandrum, India: Transworld Research Network. [Google Scholar]
- 18.Staton CA, Reed MWR, Brown NJ. 2009. A critical analysis of current in vitro and in vivo angiogenesis assays. Int. J. Exp. Pathol. 90, 195–221. ( 10.1111/j.1365-2613.2008.00633.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van Loon JJWA, Krause J, Cunha H, Gonçalves J, Almeida H, Schiller P. 2008. The large diameter centrifuge, LDC, for life and physical sciences and technology. In Life in Space for Life on Earth Symp., ESA SP-663, (ed. L Ouwehand). Angers, France: ESA Communication Production Office.
- 20.van Loon JJWA, Folgering EHTE, Bouten CVC, Veldhuijzen JP, Smit TH. 2003. Inertial shear forces and the use of centrifuges in gravity research. What is the proper control? J. Biomech. Eng. 125, 342–346. ( 10.1115/1.1574521) [DOI] [PubMed] [Google Scholar]
- 21.Rocca A, et al. 2015. Barium titanate nanoparticles and hypergravity stimulation improve differentiation of mesenchymal stem cells into osteoblasts. Int. J. Nanomed. 10, 433–445. ( 10.2217/nnm.14.188) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Josso B, Burton D, Lalor B. 2005. Texture orientation and anisotropy calculation by Fourier transform and principal component analysis. Mech. Syst. Signal Process 19, 1152–1161. ( 10.1016/j.ymssp.2004.07.005) [DOI] [Google Scholar]
- 23.Koyama T, Kimura C, Hayashi M, Watanabe M, Karashima Y, Oike M. 2009. Hypergravity induces ATP release and actin reorganization via tyrosine phosphorylation and RhoA activation in bovine endothelial cells. Eur. J. Physiol. 457, 711–719. ( 10.1007/s00424-008-0544-z) [DOI] [PubMed] [Google Scholar]
- 24.Ciofani G, Ricotti L, Rigosa J, Menciassi A, Mattoli V, Monici M. 2012. Hypergravity effects on myoblast proliferation and differentiation. J. Biosci. Bioeng. 113, 258–261. ( 10.1016/j.jbiosc.2011.09.025) [DOI] [PubMed] [Google Scholar]
- 25.Genchi GG, Cialdai F, Monici M, Mazzolai B, Mattoli V, Ciofani G. 2015. Hypergravity stimulation enhances PC12 neuron-like cell differentiation. Biomed. Res. Int. 2015, 748121. ( 10.1155/2015/748121) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Searby ND, Steele CR, Globus RK. 2005. Influence of increased mechanical loading by hypergravity on the microtubule cytoskeleton and prostaglandin E2 release in primary osteoblasts. Am. J. Physiol. Cell Physiol. 289, C148–C158. ( 10.1152/ajpcell.00524.2003) [DOI] [PubMed] [Google Scholar]
- 27.Rubenstein DA, Yin W. 2014. Hypergravity and hypobaric hypoxic conditions promote endothelial cell and platelet activation. High Alt. Med. Biol. 15, 396–405. ( 10.1089/ham.2013.1139) [DOI] [PubMed] [Google Scholar]
- 28.Versari S, Villa A, Bradamante S, Maier JAM. 2007. Alterations of the actin cytoskeleton and increased nitric oxide synthesis are common features in human primary endothelial cell response to changes in gravity. Biochim. Biophys. Acta 1773, 1645–1652. ( 10.1016/j.bbamcr.2007.05.014) [DOI] [PubMed] [Google Scholar]
- 29.Szulcek R, van Bezu J, Boonstra J, van Loon JJWA, van Nieuw Amerongen GP. 2015. Transient intervals of hyper-gravity enhance endothelial barrier integrity: impact of mechanical and gravitational forces measured electrically. PLoS ONE 10, e0144269 ( 10.1371/journal.pone.0144269) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang Y, Sang C, Paulsen K, Arenz A, Zhao Z, Jia X, Ullrich O, Zhuang F. 2010. ICAM-1 expression and organization in human endothelial cells is sensitive to gravity. Acta Astronaut. 67, 1073–1080. ( 10.1016/j.actaastro.2010.06.027) [DOI] [Google Scholar]
- 31.Auerbach R, Lewis R, Shinners B, Kubai L, Akhtar N. 2003. Angiogenesis assays: a critical overview. Clin. Chem. 49, 32–40. ( 10.1373/49.1.32) [DOI] [PubMed] [Google Scholar]
- 32.Staton CA, Stribbling SM, Tazzyman S, Hughes R, Brown NJ, Lewis CE. 2004. Current methods for assaying angiogenesis in vitro and in vivo. Int. J. Exp. Pathol. 85, 233–248. ( 10.1111/j.0959-9673.2004.00396.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Spinsi E, Bianco MC, Griffoni C, Toni M, D'Angelo R, Santi S, Riccio M, Tomasi V. 2003. Mechanosensing role of caveolae and caveolar constituents in human endothelial cells. J. Cell Phisiol. 197, 198–204. ( 10.1002/jcp.10344) [DOI] [PubMed] [Google Scholar]
- 34.Even-Ram S, Yamada KM. 2005. Cell migration in 3D matrix. Curr. Opin. Cell Biol. 17, 524–532. ( 10.1016/j.ceb.2005.08.015) [DOI] [PubMed] [Google Scholar]
- 35.Branco da Cunha C, Klumpers DD, Li WA, Koshy ST, Weaver JC, Chaudhuri O, Granja PL, Mooney DJ. 2014. Influence of the stiffness of three-dimensional alginate/collagen-I interpenetrating networks on fibroblast biology. Biomaterials 35, 8927–8936. ( 10.1016/j.biomaterials.2014.06.047) [DOI] [PubMed] [Google Scholar]
- 36.Maia FR, Fonseca KB, Rodrigues G, Granja PL, Barrias CC. 2014. Matrix-driven formation of mesenchymal stem cell–extracellular matrix microtissues on soft alginate hydrogels. Acta Biomater. 10, 3197–3208. ( 10.1016/j.actbio.2014.02.049) [DOI] [PubMed] [Google Scholar]
- 37.Shi F, Wang Y-C, Zhao T-Z, Zhang S, Du T-Y, Yang C-B, Li Y-H, Sun X-Q. 2012. Effects of simulated microgravity on human umbilical vein endothelial cell angiogenesis and role of the PI3 K-Akt-eNOS signal pathway. PLoS ONE 7, e40365 ( 10.1371/journal.pone.0040365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Costa-Almeida R, Gomez-Lazaro M, Ramalho C, Granja PL, Soares R, Guerreiro SG. 2015. Fibroblast–endothelial partners for vascularization strategies in tissue engineering. Tissue Eng Part A 21, 1055–1065. ( 10.1089/ten.tea.2014.0443) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Costa-Almeida R, Granja PL, Soares R, Guerreiro SG. 2014. Cellular strategies to promote vascularisation in tissue engineering applications. Eur. Cell Mater. 28, 51–67. [DOI] [PubMed] [Google Scholar]
- 40.Guerreiro SG, Oliveira MJ, Barbosa MA, Soares R, Granja PL. 2014. Neonatal human dermal fibroblasts immobilized in RGD-alginate induce angiogenesis. Cell Transplant. 23, 945–957. ( 10.3727/096368913X670183) [DOI] [PubMed] [Google Scholar]