Abstract
Mothers can shape the developmental trajectory of their offspring through the transmission of resources such as hormones, antioxidants or immunoglobulins. Over the last two decades, an abundant literature on maternal effects in birds has shown that several of these compounds (i.e. androgens, glucocorticoids and antioxidants) often influence the same offspring phenotypic traits (i.e. growth, immunity or oxidative stress levels), making interaction effects between egg components a likely scenario. However, the potential interactive effects of maternally transmitted compounds on offspring development and potential co-adjustment of these compounds within an egg are still poorly understood. Here, we report the results of an interspecific comparative analysis on birds' egg yolk composition (i.e. androgens and antioxidants) where we found that yolk carotenoid and vitamin E concentrations are positively associated, supporting the hypothesis that these two antioxidants act in synergy. The concentrations of vitamin E also increased with increasing concentrations of testosterone. This last result confirms the emerging idea that androgens and antioxidants are co-adjusted within eggs and that maternally transmitted antioxidants might limit the potential direct and indirect effects of prenatal exposure to high testosterone levels on oxidative stress.
Keywords: androgens, antioxidants, maternal effects, oxidative stress
1. Introduction
Prenatal conditions are known to strongly influence developmental trajectories, and to have organizational effects that can last until adulthood [1–3]. Mothers can strongly influence these prenatal conditions through the transmission of resources, such as hormones [4], antioxidants [5] or immunoglobulins [6]. The importance of these maternally transmitted compounds in transgenerational developmental plasticity has been extensively studied in various taxa and especially in birds [7], showing that several of them (e.g. androgens, glucocorticoids and antioxidants) often influence the same offspring phenotypic traits (e.g. growth, immunity or oxidative stress levels). Whether and how these maternally transmitted components may have interactive effects and are co-adjusted within eggs remains however poorly understood.
In birds, prenatal exposure to high levels of androgens (i.e. testosterone or androstenedione) stimulates faster growth and increases the vulnerability to oxidative stress [8,9]. Maternally transmitted antioxidants such as carotenoids or vitamin E, by scavenging the reactive oxygen species (ROS) produced during development [10] and/or stimulating the set-up of an efficient antioxidant system [3], might limit the consequences of this increased level of oxidative stress. In line with this hypothesis, a positive correlation between levels of yolk testosterone and antioxidants has been found in house finches (Haemorhous mexicanus) [11], suggesting that mothers co-adjust the deposition of these components in eggs. By contrast, levels of yolk testosterone and antioxidants were negatively correlated in lesser black-backed gulls, Larus fuscus [12]. More recently, an experimental approach used egg injections to manipulate yolk testosterone and carotenoid levels in Japanese quail (Coturnix japonica). Injections of either testosterone or carotenoid caused a reduction of the hatching mass and an increase of reactive oxygen metabolite (ROM) levels in chicks. However, when both egg compounds were manipulated simultaneously, these effects disappeared, suggesting that both antioxidants and androgens lose their detrimental effects when the ratio between the two compounds is balanced [3].
Based on the results of these studies, we made two main predictions. First, given that antioxidants are thought to act in synergy [13] and that carotenoids (i.e. beta-carotene) recycle vitamin E in vitro [14], enhancing the antioxidant potential of vitamin E, we predicted a positive relationship between yolk carotenoids and vitamin E levels. Second, under the hypothesis that antioxidants limit the negative effects of an exposure to high levels of testosterone on oxidative stress levels [3], we also predicted that yolk antioxidant levels should increase with increasing levels of yolk androgens. We tested these predictions in a comparative analysis of the yolk biochemical composition of bird species for which data on yolk antioxidant (i.e. carotenoids and vitamin E [15]) and androgen (i.e. testosterone and androstenedione [16] (hereafter A4)) concentrations were available.
2. Material and methods
Egg yolk concentrations in antioxidants (carotenoids and vitamin E) were available for 112 bird species from Biard et al. [15], and yolk concentrations in androgens (testosterone and A4) for 101 species from Gil et al. [16], including 78 species that were common to the two datasets (see the electronic supplementary material). It should be noted that antioxidants and androgens were measured in different eggs. To test for associations between concentrations in yolk components across bird species, we built phylogenetic linear mixed models with Markov chain Monte Carlo techniques using the R package MCMCglmm. This allowed us to control for possible phylogenetic effects, using the phylogeny from Jetz et al. ([17]; see the electronic supplementary material). We first tested for an association between the two antioxidants (carotenoids and vitamin E) using concentration of carotenoid as response variable, and concentration of vitamin E as fixed effect. Then, we tested for an association between the two androgens, including the concentration of testosterone as response variable, and of A4 as fixed effect. Finally, we tested for associations between antioxidants and androgens, including either carotenoid or vitamin E concentration as response variables, and either testosterone or A4 as fixed effects. Because clutch size, egg mass and adult body mass have been shown to predict yolk androgen and/or antioxidant concentrations [15,16], we included them as covariables in our models (using data from Myhrvold et al. [18], Dunning [19] and Jetz et al. [20]). Clutch size was not available for two species and egg mass for another one, yielding a total of 75 species for which all data were available. Note that because egg mass and adult body mass were strongly correlated (Pearson correlation coefficient = 0.987; p < 0.001), we built two models for each comparison, considering either egg mass or adult body mass. These models yielded similar results, and models including body mass are thus presented in the electronic supplementary material.
3. Results
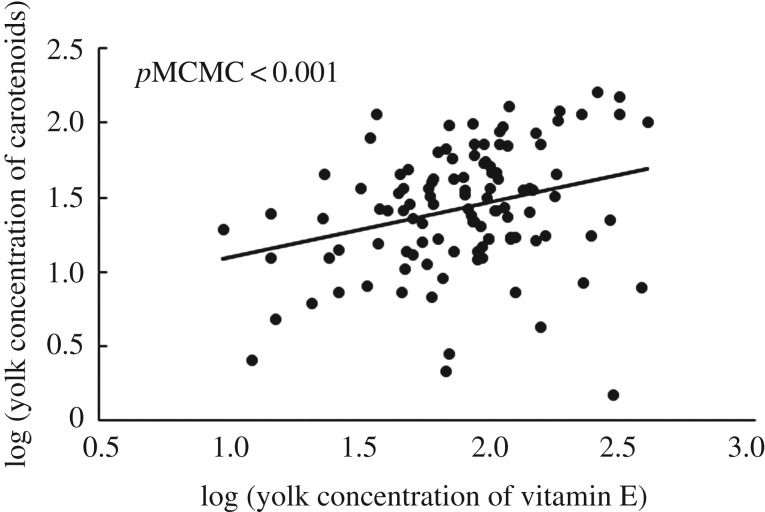
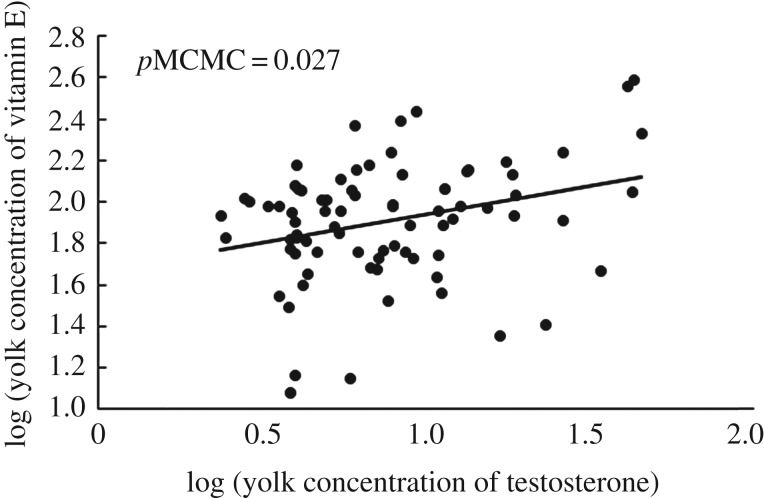
Yolk carotenoid and vitamin E concentrations were positively associated (table 1 and figure 1), as were yolk testosterone and A4 concentrations (table 1; electronic supplementary material, figure S1). Carotenoid concentration was not associated with either testosterone or A4 concentration. Finally, the concentration of vitamin E increased with the concentration of testosterone (figure 2) but not A4. Clutch size and egg mass did not affect carotenoid, vitamin E or testosterone concentration (table 1).
Table 1.
Models testing for interspecific associations between yolk concentrations of carotenoids and vitamin E, and between yolk concentrations of testosterone and A4 (androstenedione) (a), and models testing for interspecific associations between yolk concentrations of antioxidants (carotenoids and vitamin E) and androgens (testosterone and A4) (b). We used the MCMCglmm R package and included phylogeny as random factor. See the main text for details. pm, posterior mean; CI, credibility interval.
| response variable | explanatory variables | pm | CI | pMCMC | n |
|---|---|---|---|---|---|
| (a) | |||||
| carotenoid concentration | vitamin E | 0.502 | [0.282; 0.726] | <0.001 | 103 |
| clutch size | 0.049 | [−0.046; 0.145] | 0.324 | ||
| egg mass | −0.001 | [−0.002; 0.002] | 0.918 | ||
| testosterone | A4 | 0.268 | [0.115; 0.420] | 0.001 | 98 |
| clutch size | −0.028 | [−0.092; 0.035] | 0.394 | ||
| egg mass | 0.000 | [−0.001; 0.002] | 0.640 | ||
| (b) | |||||
| carotenoid concentration | testosterone | 0.125 | [−0.192; 0.443] | 0.436 | 75 |
| clutch size | 0.086 | [−0.017; 0.194] | 0.103 | ||
| egg mass | −0.000 | [−0.002; 0.002] | 0.897 | ||
| carotenoid concentration | A4 | −0.040 | [−0.295; 0.211] | 0.758 | 75 |
| clutch size | 0.087 | [−0.020; 0.192] | 0.111 | ||
| egg mass | −0.000 | [−0.002; 0.002] | 0.942 | ||
| vitamin E | testosterone | 0.264 | [0.038; 0.490] | 0.027 | 75 |
| clutch size | 0.064 | [−0.021; 0.149] | 0.139 | ||
| egg mass | −0.000 | [−0.001; 0.001] | 0.473 | ||
| vitamin E | A4 | 0.063 | [−0.127; 0.255] | 0.515 | 75 |
| clutch size | 0.070 | [−0.018; 0.158] | 0.119 | ||
| egg mass | −0.000 | [−0.001; 0.001] | 0.619 |
Figure 1.
Association between egg yolk concentration of carotenoids and vitamin E (in micrograms per gram) in 103 bird species. pMCMC comes from a model including clutch size and egg mass as covariables, and phylogeny as random variable (see the text and table 1 for details).
Figure 2.
Association between egg yolk concentration in vitamin E (in microgram per gram) and testosterone (in micrograms per gram) in 75 bird species. pMCMC comes from a model including clutch size and egg mass as covariables, and phylogeny as random variable (see the text and table 1 for details).
4. Discussion
Our study provides two key results. First, the concentrations of the two antioxidants considered here (vitamin E and carotenoids) were positively correlated. Second, the levels of yolk vitamin E were also positively associated with the levels of testosterone.
The finding that species investing more carotenoids in their eggs also allocate more vitamin E is in accordance with the fact that antioxidants are connected to each other and act in synergy. When neutralizing ROS, vitamin E is turned into a radical, which is then reduced and repaired by carotenoid molecules [21]. Thus, an increase in the concentration of vitamin E beyond the levels that the carotenoid pool can effectively recycle would reduce the overall antioxidant system effectiveness [10,22]. Surprisingly, the consequences of variations in the levels of these maternally transmitted antioxidants on chick development and then on the adult phenotype have been seldom considered in the literature. The few studies that experimentally examined this question manipulated the concentration of one antioxidant in isolation through egg injections [3,5,23–26]. Other studies used dietary carotenoid supplementation of females to indirectly manipulate levels of yolk antioxidants [27–29]. Results of these manipulations are however difficult to interpret as they may induce other modifications of mothers' physiology. Our results, though at the inter-specific level, suggest that these experiments might have disturbed the balance between antioxidants, and call for simultaneous manipulations of the entire pool of antioxidants (i.e. vitamins A, C and E, carotenoids) within the natural range of variation.
We also found that the concentrations of yolk vitamin E were positively associated with the levels of testosterone transferred by the mothers to their eggs. Recent evidence suggests that higher concentrations of yolk testosterone might directly or indirectly (i.e. through an increased growth rate [30]) impair antioxidant defences and increase the production of reactive oxygen and nitrogen species [8,9]. For example, chicks that have hatched from testosterone-injected eggs suffered from reduced plasma antioxidant levels [8] (zebra finch, Taeniopygia guttata) and DNA damage repair efficiency in response to an oxidative challenge [9] (domestic chickens, Gallus gallus). Our result suggests that species with a fast growth stimulated by high levels of yolk testosterone might counterbalance the negative effects of this developmental strategy on oxidative stress levels by allocating high levels of antioxidants, and particularly vitamin E, to their eggs. In line with this hypothesis, faster-developing species allocate higher levels of carotenoids and vitamin E to their eggs [31].
To conclude, our study shows for the first time that maternally transmitted androgens and antioxidants are co-adjusted within eggs across species, suggesting that these compounds interact with each other to shape the offspring phenotype. Future studies should analyse egg yolk composition for a suite of molecules such as hormones (androgens, glucocorticoids and thyroid hormones), antioxidants and antibodies and immunoglobulins in species with various life-history traits in order to improve our understanding of the evolution of maternal effects and of the inter-relationships between these molecules.
Supplementary Material
Acknowledgements
We thank Clothilde Biard and Diego Gil for making their datasets available, Aurelie Boudier for help with data gathering and three anonymous reviewers for helpful comments on the manuscript.
Data accessibility
Data are available from the Dryad Digital Repository [32]: http://dx.doi.org/10.5061/dryad.7gk6d.
Authors' contributions
M.G. designed the study. S.D. ran the statistical analyses. M.G. and S.D. wrote the manuscript. Both authors agree to be held accountable for the content herein and gave final approval for publication.
Competing interests
We declare we have no competing interests.
Funding
This paper is part of the project CGL2013-47448-P from the Spanish Government to Daniel Sol.
References
- 1.Mousseau TA, Fox CW. 1998. Maternal effects as adaptations. New York, NY: Oxford University Press. [Google Scholar]
- 2.Groothuis TGG, Wendt M, von Engelhardt N, Carere C, Eising C. 2005. Maternal hormones as a tool to adjust offspring phenotype in avian species. Neurosci. Biobehav. Rev. 29, 329–352. ( 10.1016/j.neubiorev.2004.12.002) [DOI] [PubMed] [Google Scholar]
- 3.Giraudeau M, Ziegler AK, Pick JL, Ducatez S, Canale CI, Tschirren B. 2016. Interactive effects of yolk testosterone and carotenoid on prenatal growth and offspring physiology in a precocial bird. Behav. Ecol. 27, rw127. ( 10.1093/beheco/arw127) [DOI] [Google Scholar]
- 4.Schwabl H. 1993. Yolk is a source of maternal testosterone for developing birds. Proc. Natl Acad. Sci. USA 90, 11 446–11 450. ( 10.1073/pnas.90.24.11446) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Romano M, Caprioli M, Ambrosini R, Rubolini D, Fasola M, Saino N. 2008. Maternal allocation strategies and differential effects of yolk carotenoids on the phenotype and viability of yellow-legged gull (Larus michahellis) chicks in relation to sex and laying order. J. Evol. Biol. 21, 1626–1640. ( 10.1111/j.1420-9101.2008.01599.x) [DOI] [PubMed] [Google Scholar]
- 6.Gasparini J, McCoy KD, Haussy C, Tveraa T, Boulinier T. 2001. Induced maternal response to the Lyme disease spirochaete Borrelia burgdorferi sensu lato in a colonial seabird, the kittiwake Rissa tridactyla. Proc. R. Soc. Lond. B 268, 647–650. ( 10.1098/rspb.2000.1411) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Uller T. 2008. Developmental plasticity and the evolution of parental effects. Trends Ecol. Evol. 23, 432–438. ( 10.1016/j.tree.2008.04.005) [DOI] [PubMed] [Google Scholar]
- 8.Tobler M, Sandell MI. 2009. Sex-specific effects of prenatal testosterone on nestling plasma antioxidant capacity in the zebra finch. J. Exp. Biol. 212, 89–94. ( 10.1242/jeb.020826) [DOI] [PubMed] [Google Scholar]
- 9.Treidel LA, Whitley BN, Benowitz-Fredericks ZM, Haussmann MF. 2013. Prenatal exposure to testosterone impairs oxidative damage repair efficiency in the domestic chicken (Gallus gallus). Biol. Lett. 9, 20130684 ( 10.1098/rsbl.2013.0684) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Surai PF. 2002. Natural antioxidants in avian nutrition and reproduction. Nottingham, UK: Nottingham University Press. [Google Scholar]
- 11.Navara KJ, Badyaev AV, Mendonça MT, Hill GE. 2006. Yolk antioxidants vary with male attractiveness and female condition in the house finch (Carpodacus mexicanus). Physiol. Biochem. Zool. 79, 1098–1105. ( 10.1086/507661) [DOI] [PubMed] [Google Scholar]
- 12.Royle NJ, Surai PF, Hartley PF. 2001. Maternally derived androgens and antioxidants in bird eggs: complementary but opposing effects? Behav. Ecol. 12, 381–385. ( 10.1093/beheco/12.4.381) [DOI] [Google Scholar]
- 13.Catoni C, Peters A, Schaefe M. 2008. Life-history trade-offs are influenced by the diversity, availability and interactions of dietary antioxidants. Anim. Behav. 76, 1107–1119. ( 10.1016/j.anbehav.2008.05.027) [DOI] [Google Scholar]
- 14.Bohm F, Edge R, Land EJ, McGarvey DJ, Truscott TG. 1997. Carotenoids enhance vitamin E antioxidant efficiency. J. Am. Chem. Soc. 119, 621–622. ( 10.1021/ja962512c) [DOI] [Google Scholar]
- 15.Biard C, Gil D, Karadaş F, Saino N, Spottiswoode CN, Surai PF, Møller AP. 2009. Maternal effects mediated by antioxidants and the evolution of carotenoid-based signals in birds. Am. Nat. 174, 696–708. ( 10.1086/606021) [DOI] [PubMed] [Google Scholar]
- 16.Gil D, Biard C, Lacroix A, Spottiswoode CN, Saino N, Puerta M, Møller AP. 2007. Evolution of yolk androgens in birds: development, coloniality, and sexual dichromatism. Am. Nat. 169, 802–819. ( 10.1086/516652) [DOI] [PubMed] [Google Scholar]
- 17.Jetz W, Thomas GH, Joy JB, Hartmann K, Mooers AO. 2012. The global diversity of birds in space and time. Nature 491, 444–448. ( 10.1038/nature11631) [DOI] [PubMed] [Google Scholar]
- 18.Myhrvold NP, Baldridge E, Chan B, Sivam D, Freeman DL, Ernest SKM. 2015. An amniote life-history database to perform comparative analyses with birds, mammals, and reptiles. Ecology 96, 3109 ( 10.1890/15-0846R.1) [DOI] [Google Scholar]
- 19.Dunning JB. 2007. CRC handbook of avian body masses, 2nd edn Boca Raton, FL: CRC Press. [Google Scholar]
- 20.Jetz W, Sekercioglu CH, Böhning-Gaese K. 2008. The worldwide variation in avian clutch size across species and space. PLoS Biol. 6, e303 ( 10.1371/journal.pbio.0060303) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Costantini D. 2008. Oxidative stress in ecology and evolution: lessons from avian studies. Ecol. Lett. 11, 1238–1251. ( 10.1111/j.1461-0248.2008.01246.x) [DOI] [PubMed] [Google Scholar]
- 22.Costantini D, Coluzza C, Fanfani A, Dell'Omo G. 2007. Effects of carotenoid supplementation on colour expression, oxidative stress and body mass in rehabilitated captive adult kestrels (Falco tinnunculus). J. Comp. Physiol. B 177, 723–731. ( 10.1007/s00360-007-0169-0) [DOI] [PubMed] [Google Scholar]
- 23.Saino N, Romano M, Caprioli M, Rubolini D, Ambrosini R. 2011. Yolk carotenoids have sex-dependent effects on redox status and influence the resolution of growth trade-offs in yellow-legged gull chicks. Behav. Ecol. 22, 411–421. ( 10.1093/beheco/arq220) [DOI] [Google Scholar]
- 24.Møller AP, Biard C, Karadas F, Rubolini D, Saino N, Surai PF. 2011. Maternal effects and changing phenology of bird migration. Clim. Res. 49, 201–210. ( 10.3354/cr01030) [DOI] [Google Scholar]
- 25.Parolini M, Romano M, Caprioli M, Rubolini D, Saino N. 2015. Vitamin E deficiency in last-laid eggs limits growth of yellow-legged gull chicks. Funct. Ecol. 29, 1070–1077. ( 10.1111/1365-2435.12412) [DOI] [Google Scholar]
- 26.Giraudeau M, Ziegler AK, Tschirren B. 2016. Long-term effect of yolk carotenoid levels on testis size in a precocial bird. Biol. Lett. 12, 20160008 ( 10.1098/rsbl.2016.0008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Koutsos EA, Clifford AJ, Calvert CC, Klasing KC. 2003. Maternal carotenoid status modifies the incorporation of dietary carotenoids into immune tissues of growing chickens (Gallus gallus domesticus). J. Nutr. 133, 1132–1138. [DOI] [PubMed] [Google Scholar]
- 28.McGraw KJ, Adkins-Regan E, Parker RS. 2005. Maternally derived carotenoid pigments affect offspring survival, sex ratio, and sexual attractiveness in a colorful songbird. Naturwissenschaften 92, 375–380. ( 10.1007/s00114-005-0003-z) [DOI] [PubMed] [Google Scholar]
- 29.Grether GF, Kolluru GR, Lin K, Quiroz MA, Robertson G, Snyder AJ. 2008. Maternal effects of carotenoid consumption in guppies (Poecilia reticulata). Funct. Ecol. 22, 294–302. ( 10.1111/j.1365-2435.2007.01365.x) [DOI] [Google Scholar]
- 30.Schwabl H, Palacios MG, Martin TE. 2007. Selection for rapid embryo development correlates with embryo exposure to maternal androgens among passerine birds. Am. Nat. 170, 196–206. ( 10.1086/519397) [DOI] [PubMed] [Google Scholar]
- 31.Deeming DC, Pike TW. 2013. Embryonic growth and antioxidant provision in avian eggs. Biol. Lett. 9, 20130757 ( 10.1098/rsbl.2013.0757) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Giraudeau M, Ducatez S. 2016. Data from: Co-adjustment of yolk antioxidants and androgens in birds. Dryad Digital Repository. ( 10.5061/dryad.7gk6d) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from the Dryad Digital Repository [32]: http://dx.doi.org/10.5061/dryad.7gk6d.