Extended Data Figure 8. Rationalization of PAM-2 and PAM-3 specificity using nucleotide substitution and modeling.
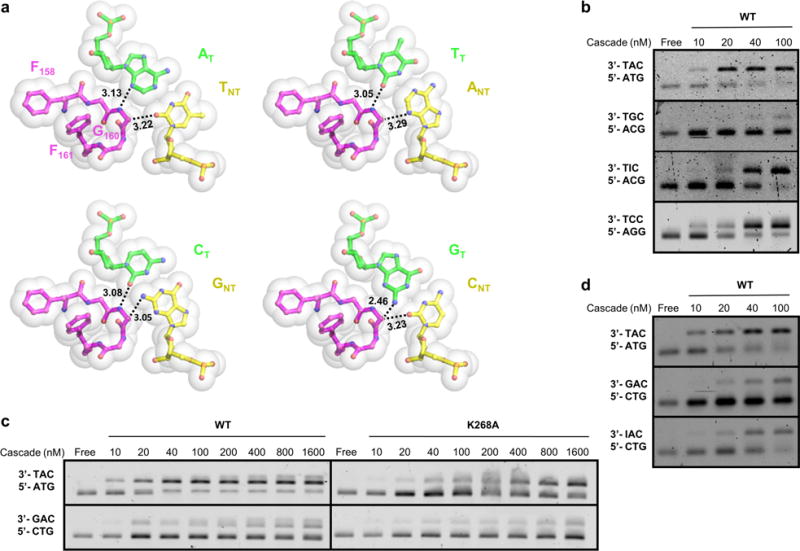
a, Modeling of alternative base-pairs at PAM-2 suggests that only the N2 amine of GT-2 would cause steric clashes with Cα of G160 (lower right quadrant), this amine therefore may serve as the anti-determinant for the rejection of GT-CNT at PAM-2. b, EMSAs demonstrating that removal of this amine in inosine substitution rescued the Cascade binding defect. c. Whereas K268A contained reduced affinity for the correct PAM, it still possessed strong discrimination against GT-CNT at PAM-3 (5′-CTG), suggesting the further presence of a mechanism to reject GT-3. d, Inosine substitution of GT-3 restored the Cascade binding Kd to ~40 nM, leading to the conclusion that the N2 amine of GT-3 is a minor determinant of specificity.
