Fig. 3.
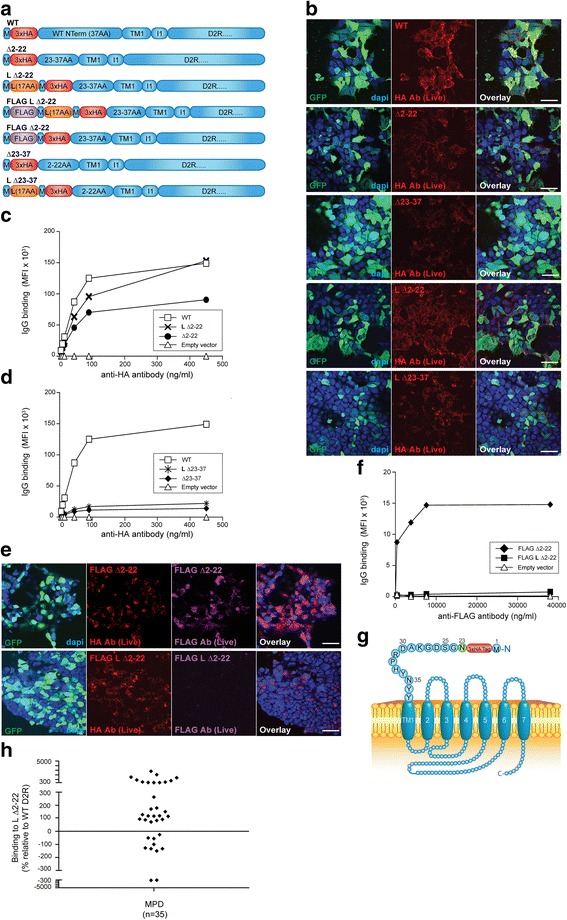
The majority of patient antibodies bind to a region encompassing amino acids 23-37 of D2R extracellular N-terminus. a Open reading construct of WT D2R, Δ2-22 (D2R missing amino acids 2-22 of extracellular N-terminus), L Δ2-22 (Δ2-22 with additional cleavable Lucy (L) signal peptide), FLAG L Δ2-22, FLAG Δ2-22, Δ23-37 D2R (D2R missing amino acids 23-37 of D2R extracellular N-terminus), L Δ23-37 D2R (Δ23-37 with additional cleavable L signal peptide). M = methionine; AA = amino acid; Red = 3x haemagglutinin (HA) tag; Blue = D2R sequence; Purple = FLAG sequence; TM1 = first transmembrane domain; I1 = first intracellular loop; Green = N-glycosylation site. b Confocal images after live immunolabeling of WT D2R and mutant-transfected HEK293 cells using an anti-HA antibody showed decreased cell surface expression of Δ2-22 and Δ23-37 compared to WT D2R. Introduction of a L signal peptide in Δ2-22, but not in Δ23-37, significantly increased surface expression to levels observed in WT D2R (scale bar = 50 μm). c Increase of surface expression on live L Δ2-22-transfected HEK293 cells was confirmed by flow cytometry. Representative data out of three independent experiments is shown. d Lack of rescue of surface expression on live L Δ23-37-transfected HEK293 cells was confirmed by flow cytometry. Representative data out of three independent experiments is shown. e, f Confocal imaging and flow cytometry analyses after live immunolabeling of FLAG L Δ2-22- and FLAG Δ2-22-transfected HEK293 cells showed successful L signal peptide cleavage in FLAG L Δ2-22-transfected HEK293 cells (GFP staining not shown in overlay) (scale bar = 50 μm). g Schematic of L Δ2-22 D2R at cell surface. Red = 3xHA tag; Green = N-glycosylation site; Blue = D2R sequence. h Sera from anti-D2R antibody-positive movement and psychiatric disorders (MPD, n = 35) were incubated with empty vector-, WT D2R-, and L Δ2-22-transfected live HEK293 cells at 1:50 dilution, followed by AF647-conjugated anti-human IgG secondary antibody, and flow cytometry analysis. Percentage of sera binding to L Δ2-22 (MFI %) was calculated using the formula described in Material and Methods. 71% patients (25/35) recognized L Δ2-22 (518 ± 683%, n = 25), whereas 29% (10/35) showed no immunoreactivity to amino acids 23-37. Binding threshold is represented by solid line on graph. Representative data out of three experiments is shown
