Abstract
The war on cancer has been fought during the past several decades primarily based on the somatic mutation model of cancer. This has resulted in the emphasis on cancer screening and elimination of any detected cancerous/precancerous cells as the primary method of cancer prevention. This approach has reduced mortality from some cancers, but age-adjusted cancer mortality rates continue to be high. The lack of significant progress in reducing cancer mortality rates may be indicative of a fundamental flaw in the cancer model used. An alternative model of cancer is the immune suppression model of cancer based on the tremendous increase in cancers when the immune system is suppressed. According to this model, the key carcinogenic event is the suppression of the immune system which enables the already existing covert cancers to grow uncontrollably, causing cancer. Hence, cancer screening would consist of identifying those with weak immune system response. The primary mode of cancer prevention and treatment would be boosting of the immune system, for example, through exercise, infection, and low-dose radiation, as they are all known to enhance immune system response and reduce cancers. There is sufficient evidence to justify clinical trials of this approach for cancer screening, prevention, and treatment.
Keywords: cancer screening, cancer prevention, cancer treatment, exercise, infection, low-dose radiation
Introduction
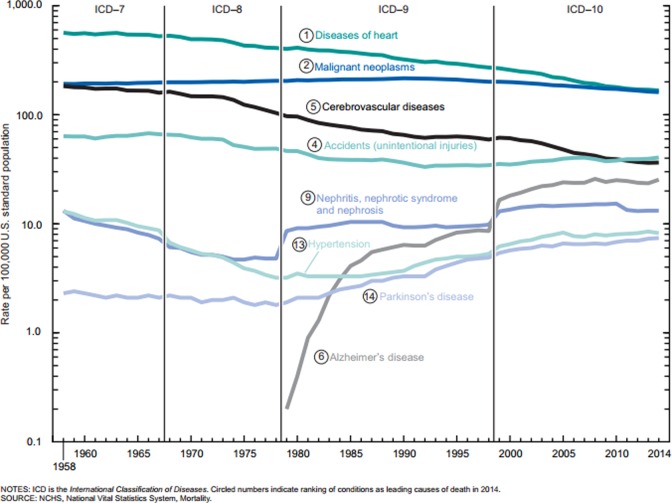
The United States declared a war on cancer with the passage and signing of the National Cancer Act of 1971 and has devoted considerable resources to conquering cancer. Global efforts against cancer have also increased tremendously during the past several decades.1 Many breakthroughs have been made in understanding the nature of cancer,2,3 and there have been major improvements in the techniques of cancer screening, prevention, diagnosis, and treatment over the years.4 However, age-adjusted cancer mortality rates continue to remain high at approximately 170 per 100 000 (Figure 1).5
Figure 1.
Age-adjusted death rates from major noncommunicable diseases in the United States from 1950 to 2010. Data from Hunter et al.5
The lack of significant progress in the cancer field has been acknowledged in recent reviews with statements such as “the current paradigm is immersed in crisis,”6 “the battle has not yet been won, despite a substantial investment in resources,”7 and “the war has not been won.”8 Although there are suggestions to continue on the present course with refinements,8 a major change in the cancer paradigm has also been recommended.6 The purpose of this review is to discuss the current cancer paradigm; identify some of the problems with the present approach to cancer screening, prevention, and treatment; and suggest a new approach for dealing with cancer based on an alternative paradigm.
Current Paradigm of Cancer Screening, Prevention, and Treatment
The somatic mutation model of cancer has been the prevailing paradigm in the cancer field for many decades.9 The transformation of a normal cell to a cancer cell through mutations is considered to be the key event in the carcinogenic process and is in fact generally referred to as carcinogenesis. This transformation has been the subject of intense study with many hallmarks of cancer being identified.2,3 The recently reported positive correlation between lifetime risk for 33 specific types of cancers and the number of stem cell divisions in the corresponding tissues10 appears to lend further support to the idea that cancer is the result of “bad luck,” that is, accumulation of random mutations.
Since the occurrence of random mutations cannot be averted, the only method of preventing the adverse impact of the resulting cancers is to detect the cancerous or precancerous cells early and eliminate them before they can multiply and metastasize since cancers detected at an early stage have a better prognosis.11 Thus, cancer screening of asymptomatic population has become the main strategy for the prevention of some of the cancers, including the most common ones.12 The results from this approach have been mixed. The mortality rate for cervical cancer has reduced considerably following the widespread adoption of Pap smear tests.13 However, for thyroid cancer, increased screening has not resulted in reducing thyroid cancer mortality.14 For breast cancer, the benefits of mammography screening are being debated.15,16 For prostate cancer, the benefits of screening are not clear and there are concerns about the harms of the treatments resulting from early detection.17
During the cancer screening process, the detection of malignant cells in biopsy is considered to be a definitive diagnosis of cancer based on the somatic mutation model of cancer and usually results in treatment(s) to eliminate the tumors through surgery, radiation therapy, and/or chemotherapy. However, early detection can also result in overdiagnosis18 and overtreatment which can harm patients19,20 since many of the cancer treatments have adverse side effects, both long term and short term, diminishing the patients’ quality of life.21 Because of the significant adverse side effects from the cancer treatments and the net harm to patients because of overdiagnosis,22,23 watchful waiting has been suggested for some cancers detected through screening.24 However, patients have major concerns with this approach,25 and the possibility that a small percentage of the detected cancers would metastasize and be fatal is of concern.
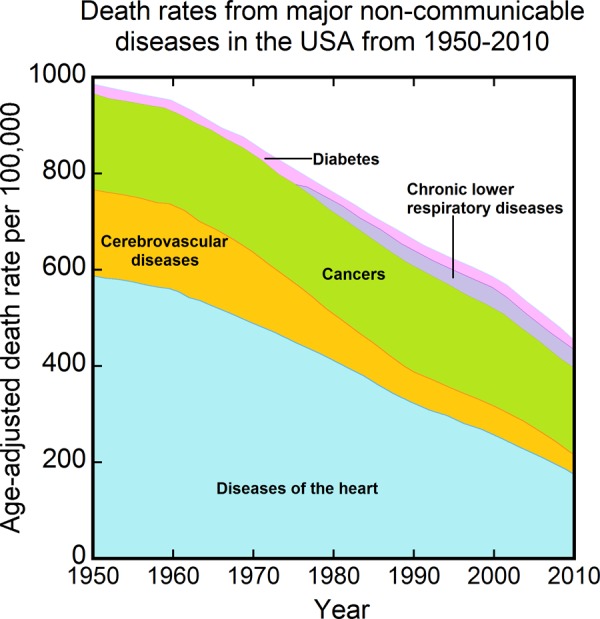
The results from the current approach to cancer have been disappointing. Cancer screening has not been effective in reducing overall cancer mortality,26 and age-adjusted cancer mortality rates have remained relatively flat in the United States during the past 50 years (Figure 2).27
Figure 2.
Age-adjusted death rates for selected leading causes of death: United States, 1958 to 2014. Data from Kochanek et al.27
Though there has been some reduction in these rates since the early 1990s, much of the reduction may be related to the decrease in smoking that began in the 1960s following the start of the smoking cessation campaign (Figure 3).28
Figure 3.
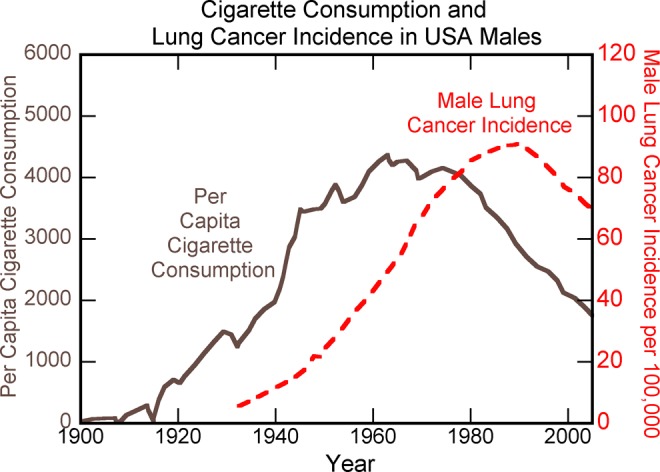
Per capita cigarette consumption and male lung cancer incidence in the United States over the last century. Data from AACR Progress Report.28
An analysis has suggested that without reductions in smoking, there would have been virtually no decrease in overall cancer mortality in either men or women since the early 1990s.29 Considering the vastly improved technologies for cancer screening, prevention, detection, and treatment that exist today compared to decades ago,4 the absence of major progress in cancer mortality rates (when the cancer reduction due to reduced smoking is excluded from consideration) indicates there may be a fundamental flaw in the current approach to cancer based on the somatic mutation model of cancer.
Problems With the Somatic Mutation Model of Cancer
There are indeed many reasons why the somatic mutation model of cancer is not valid.30,31 Some of the reasons will be discussed here. Figure 4 shows accumulated mutations in spleen of mice as a function of age.32
Figure 4.
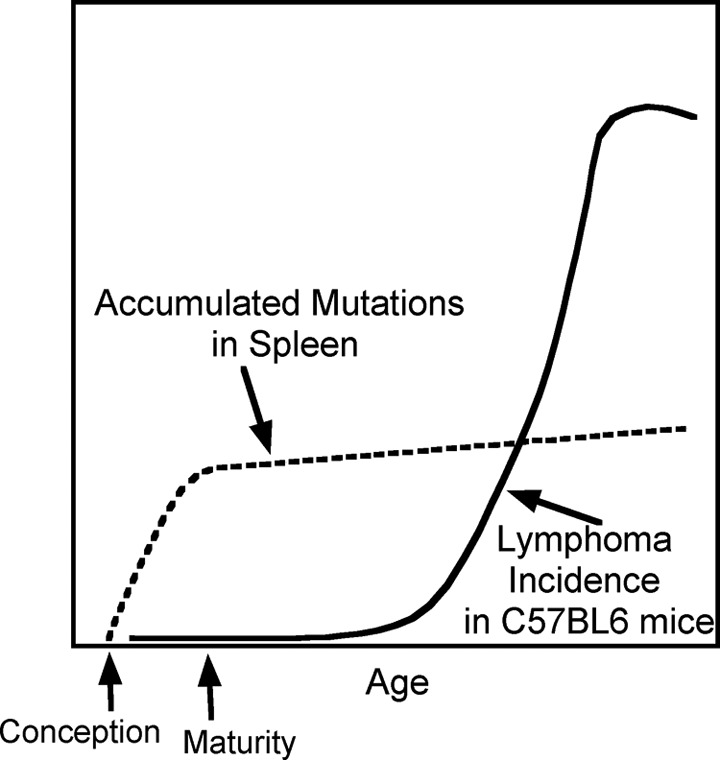
Comparison of the time courses of mutation accumulation with onset of malignancies in the hematopoietic system in mice. Stylized curves represent the numbers of mutations detected in spleens (dashed line) and lymphoma incidence (solid line) in C57BL/6 mice. Data from DeGregori.32
The maximum accumulation of mutations is seen to occur in the early period of growth between conception and maturity when cells would be dividing at high rates and so would be most vulnerable to mutations, but lymphoma rates are at the lowest levels during this period32 contradicting the somatic mutation model of cancer. For humans, although mutations would be accumulating at high rates during childhood and adolescence, cancer rates are very low during these periods (Figure 5).33
Figure 5.
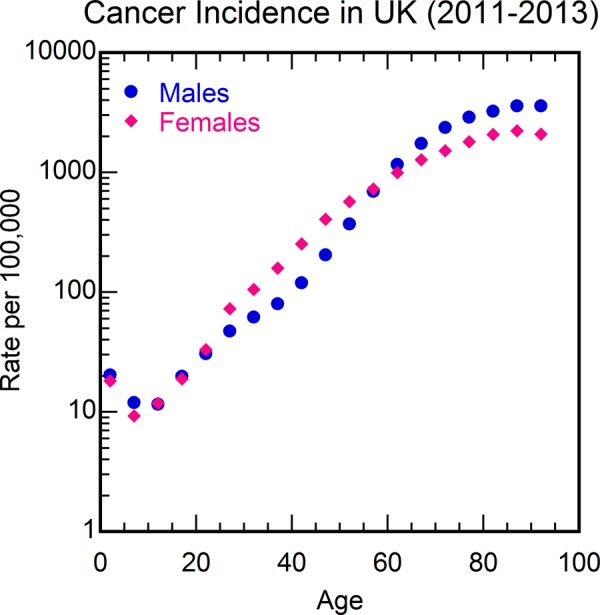
Cancer incidence rate per 100 000 in the United Kingdom from 2011 to 2013 as a function of age. Data from Cancer Research UK.33
An autopsy study has shown that the percentage of patients having cancer cells in their bodies remain more or less unchanged from ages near 50 to older ages (Figure 6),34 whereas cancer mortality rate increases by more than an order of magnitude between these age ranges (Figure 5),33 again contradicting the somatic mutation model of cancer. Autopsy studies have shown that almost everyone has covert cancers, that is, precancerous cells or cancerous cells, but lifetime risk of being diagnosed with cancer is about 30% worldwide35 indicating that cancerous mutations do not imply cancer. Even for newborns, it is estimated that 1 in 5 may have covert premalignant cancer, but cancer rate in children younger than 15 is more than 100 times lower.35 Thus, cancerous mutations do not imply cancer. In a recent study that examined a small area of skin, a heavy burden of mutations was observed in normal skin cells at a level similar to that seen in many cancers, but the patients did not have skin cancer.36 Although many carcinogens are mutagens, there are some carcinogens that are not mutagens, for example, alcohol,37 indicating that some factor other than mutations is causing the cancers. Also, there are some mutagens that are not carcinogens, for example, sodium azide,38 indicating that mutations do not imply cancer. Another feature of cancers that contradicts the mutation model of cancer is Peto’s paradox—Cancer incidence does not scale with body size across species.39 The observed spontaneous regression of tumors for several types of cancers40 also contradicts the somatic mutation mode of cancer. Finally, using the somatic mutation model of cancer, the reported increase in DNA damage from even 5 minutes of strenuous exercise41 would imply an increased risk of cancer from regular exercise. The observed reduction of cancer mortality with exercise42 is completely contradictory to this prediction.
Figure 6.
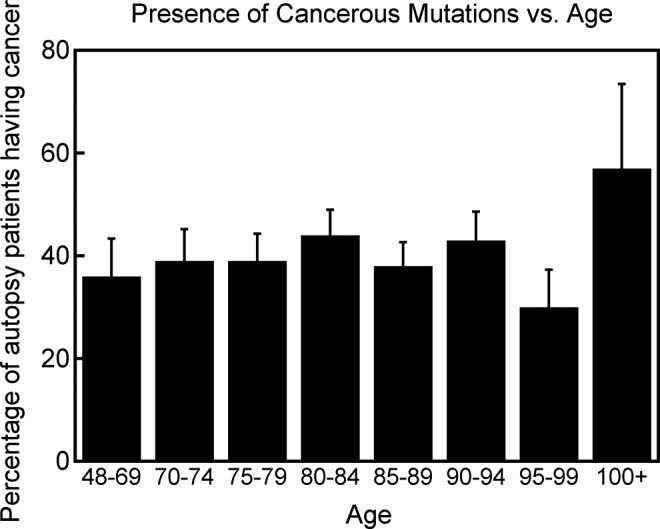
Percentage of total autopsy patients in a geriatric hospital in Japan who had cancer cells in their body as a function of age. Data from Imaida et al.34
Immune Suppression Model of Cancer
An alternative model of cancer is the immune suppression model of cancer, based on the well-known tremendous increase in cancers in patients with organ transplant and patients with HIV/AIDS in whom the immune system is suppressed (Figures 7 and 8).43,44 In this model of cancer, a normal cell, with the accumulation of mutations, can become a cancer cell. However, the immune system would prevent the uncontrolled growth of cancer cells by eliminating them or keeping them under control, resulting in covert cancer.45 When the immune system is suppressed, for example, due to aging (Figure 9),46 the covert cancers would grow uncontrollably to overt cancers. There is indeed a large amount of supportive evidence for the immune suppression model of cancer in the form of increased cancer risk observed with the weakening of the immune system and decreased cancer risk observed with the enhancement of the immune system.47 Children have the strongest immune system, and aging reduces immune system response (Figure 9)46 explaining qualitatively the well-known age-dependent increase in cancer risk (Figure 5).33
Figure 7.
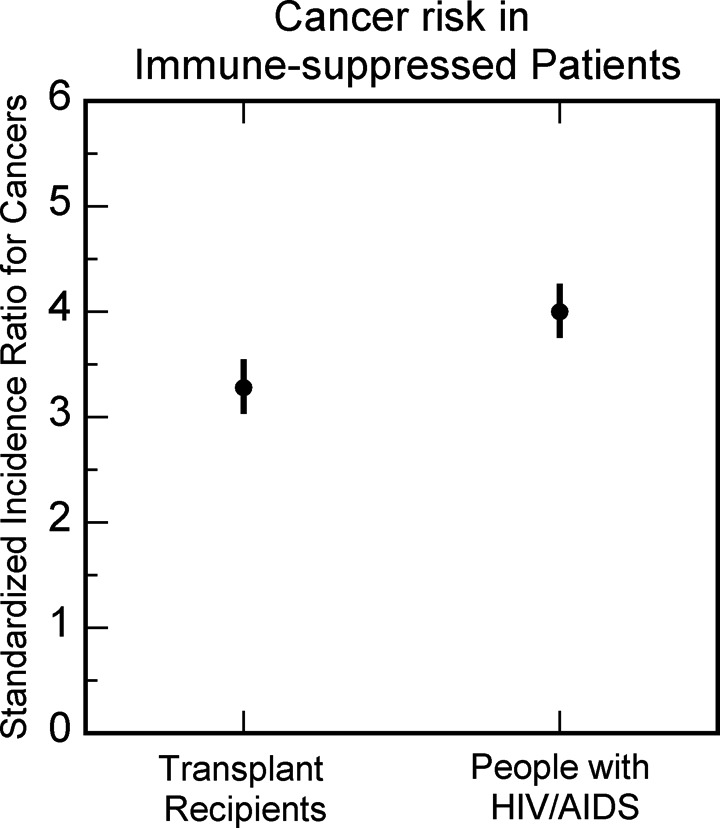
Standardized incidence ratio for cancers in organ transplant recipients and people with HIV/AIDS. Data from Oliveira Cobucci et al.43
Figure 8.
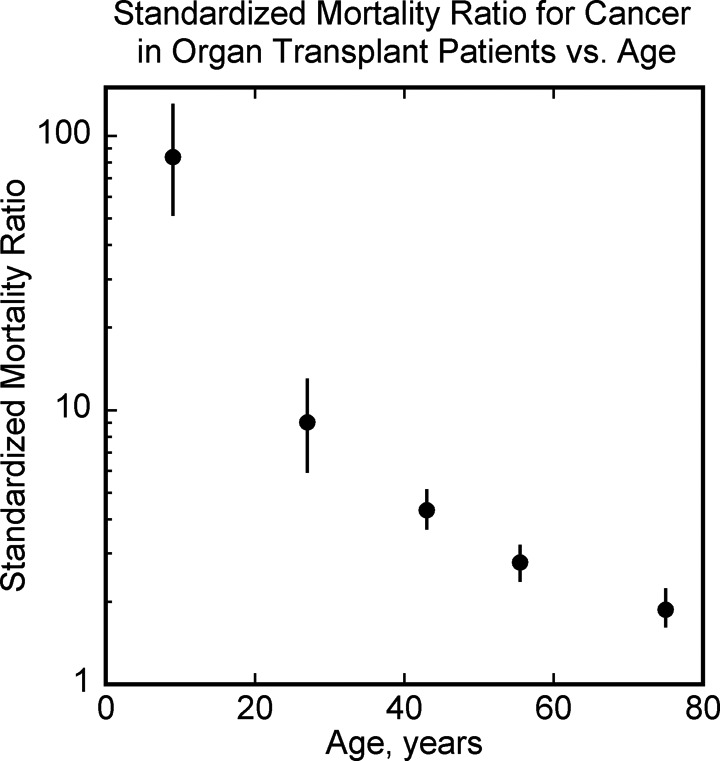
Standardized mortality ratio (SMR) for cancers as a function of age in organ transplant recipients. Data from Acuna et al.44
Figure 9.
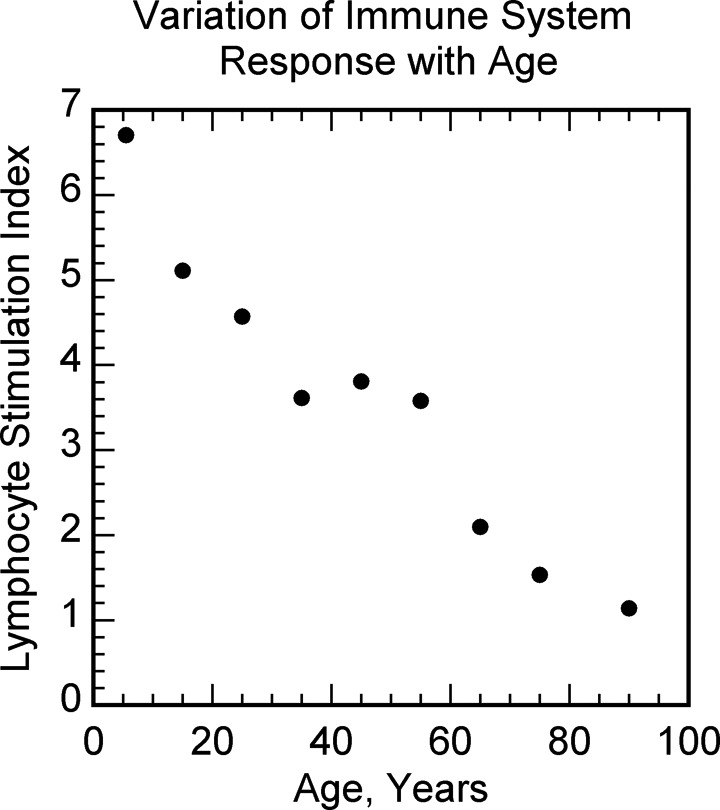
Varicella-zoster virus-specific cell-mediated immune response as a function of age. Data from Levin et al.46
Females have stronger immune system than males48 and have lower risk of cancer compared to males.49 Allergy sufferers have overactive immune system and have lower risk of cancer.50 Breastfeeding enhances the immune system in infants,51 and it reduces childhood leukemias.52 Exercise and infections enhance the immune system53,54 and they both reduce cancers.42,55 High-dose radiation, cigarettes, and alcohol suppress the immune system56-58 and they all increase cancer risk.57,59,60 Low-dose radiation stimulates the immune system (Figure 10),61 and reduced cancers have been observed in cohorts that have been exposed incidentally or accidentally to low-dose radiation (Figure 11).62-65
Figure 10.
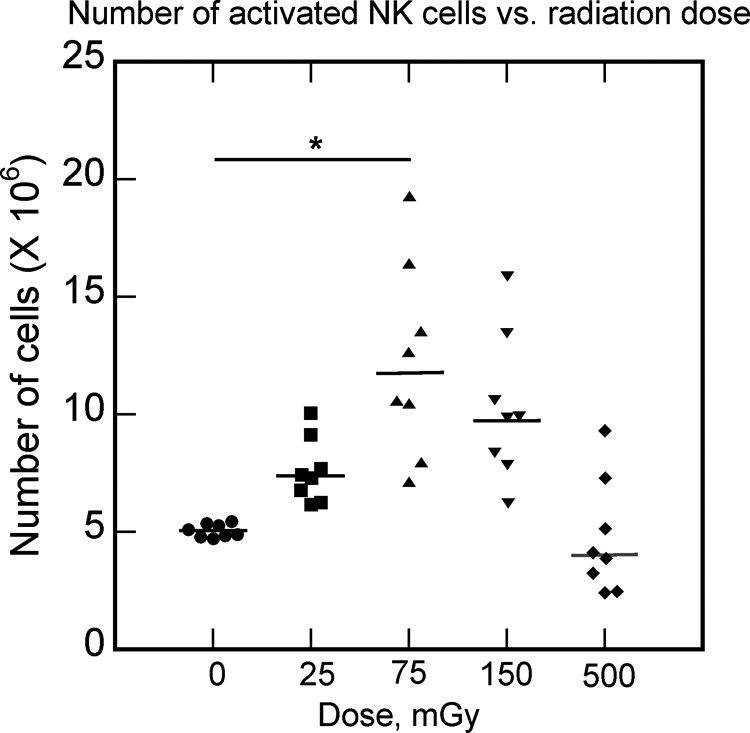
The absolute number of natural killer (NK) cells after irradiation at different doses (25, 75, 150, and 500 mGy). Data from Yang et al.61
Figure 11.
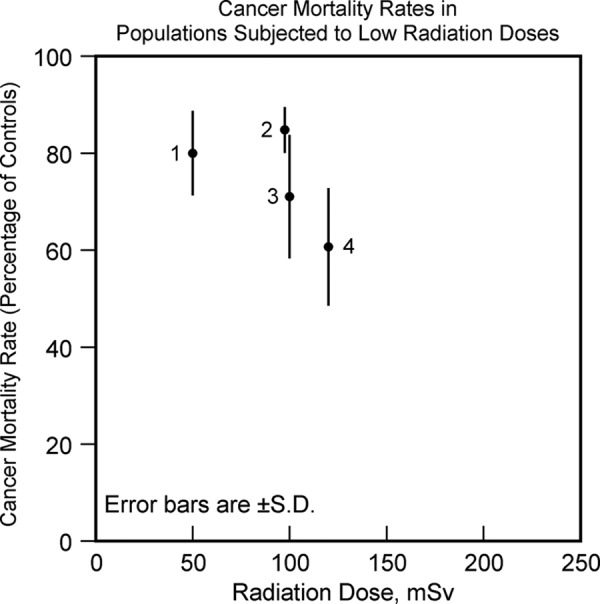
Cancer mortality rates in population groups exposed to low-dose radiation expressed as percentage of cancer mortality rate in control population versus radiation dose. Data labels—(1) residents of radio-contaminated apartment buildings in Taiwan. Data from Hwang et al,62 (2) radiation workers in Nuclear Shipyard Worker Study. Data from Sponsler and Cameron,63 (3) British radiologists who entered service during the period 1955 to 1979. Data from Berrington et al,64 and (4) evacuated residents of villages near Mayak Nuclear Weapons Facility. Data from Kostyuchenko et al.65 All the cohorts showed reduced risk of cancer compared to equivalent control populations not subjected to the radiation exposures.
All of this evidence supports the immune suppression model of cancer. However, it is indeed possible for cancers to develop in spite of the fully functioning immune system.66,67 As cancerous mutations develop, the immune system would keep the cancerous and precancerous cells under check and in equilibrium. During this equilibrium phase, cancer cells would be undergoing Darwinian evolution and phenotypes of cancer cells that evade the immune system may develop. Such cancer cells would be able to escape the control of the immune system and grow uncontrollably, causing cancer.66 However, considering the tremendous decrease in overall cancer mortality rates when the immune system is boosted, and the effectiveness of low-dose radiation cancer treatments which boost the immune system, the immune system evading cancers may not be a significant part of the cancer mix, and the suppression of the immune system is likely to be the primary cause of most cancers.
Cancer Screening, Prevention, and Treatment Under the Immune Suppression Model of Cancer
Cancer Screening
Since suppression of the immune system is the primary cause of cancers, cancer screening should consist of identifying those that have weak immune system response, as they would be the most susceptible to developing clinical cancers. Both innate and adaptive immune systems work cooperatively in eliminating cancer cells67 and so it would be useful to quantify both of these immune responses. The immune system responses may be assessed, for example, by challenging peripheral blood mononuclear cells with various agents and assaying immune system-related cytokines.68 In addition, at the time of any routine immunizations, blood samples can be collected prior to and a few weeks after the immunizations and assayed for the immune system response to the vaccinations. Those found to have weak responses in these assessments should be given interventions such as exercise, infection, and low-dose radiation to elevate their immune system responses, and the effectiveness of the interventions should be confirmed by further assays. Since these interventions would not have any major adverse side effects but would improve health in other aspects, overdiagnosis and overtreatment would not be of as much concern. Clinical trials are needed to assess the effectiveness of this approach to cancer screening.
Prevention of Cancer
Since suppression of the immune system is the primary cause of cancers, the primary method of cancer prevention would be to boost the immune system. This can be achieved, for example, via exercise, infection, and low-dose ionizing radiation since they are all known to boost the immune system. However, there are some practical issues in utilizing these interventions.
Though it is well known that exercise improves overall health and is recommended for the general population,69 a large segment of the population does not engage in much physical activity.70,71 Improving compliance with the exercise prescription in the population would be helpful in reducing cancers. The current campaigns for exercise do not emphasize the cancer preventive effect of exercise, and this beneficial effect of exercise is not well known to the public. Modifying the advertising campaign message and stressing the cancer preventive effect of regular exercise may result in better compliance with exercise recommendations.
Stimulation of the immune system through daycare infections in infants is known to reduce the risk of childhood leukemias.72 For adults also, a history of fevers due to infections has been associated with reduced cancer risk later in life.73 Whether similar cancer preventive effect may be attained by stimulating the immune systems through vaccinations should be studied.
The use of low-dose radiation for boosting the body’s defenses and preventing cancers should be explored in clinical trials since many population groups that were exposed to low-dose radiation were found to have significantly reduced cancers, as noted earlier (Figure 11). One difficulty with such use of low-dose radiation is that the observed cancer preventive effect of low-dose radiation is not well known. Since ionizing radiation is known to cause DNA damage and mutations, even a small amount of radiation is presumed to increase cancer risk, based on the invalid somatic mutation model of cancer. However, low-dose radiation would also boost the defenses in the body, such as antioxidants and DNA repair enzymes, and so would reduce the occurrence of mutations in the subsequent period, with the final result being reduced DNA damage and mutations following low-dose radiation exposures.74 This has been observed in studies with drosophila melanogaster (Figure 12)75 and mice (Figure 13).76
Figure 12.
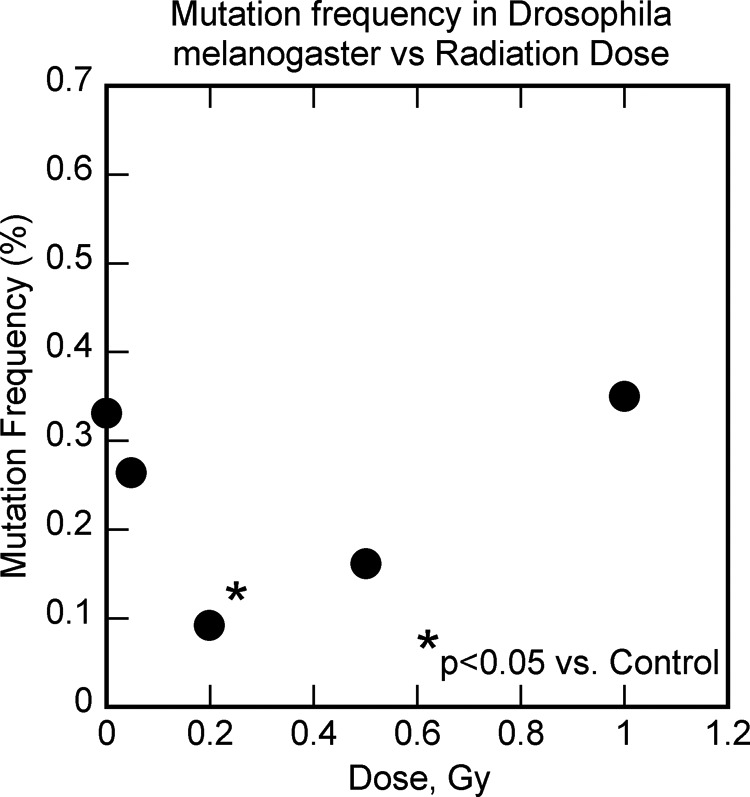
The effect of low-dose radiation on mutations in drosophila melanogaster. Data from Koana et al.75
Figure 13.
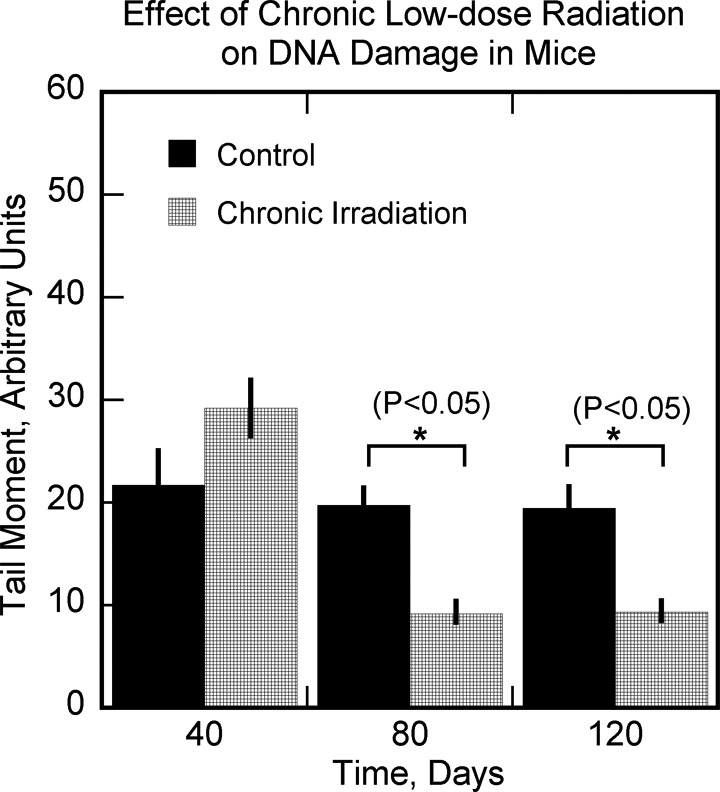
The effect of chronic low-dose radiation on DNA damage in mice. Data from Osipov et al.76
One consequence of the reduced mutations is that fewer cancer cells would be formed and would be in the equilibrium phase of the immunoediting process,66 and so there would be less chance of forming immune system-evading cancers. Another consequence of the DNA damage due to low-dose radiation exposures is that the damaged cells would upregulate retinoic acid early inducible proteins (RAE-1) and other ligands of the NKG2D receptor activating natural killer cells,77 which play an important role in eliminating tumor cells.
Other interventions that increase the immune system response such as reducing obesity,78 smoking cessation,57 fruit/vegetable diet,79 fasting,80 and so on—if they are applicable—should also be studied. The boosted immune system from all these interventions would eliminate the precancerous and cancerous cells more efficiently, reducing the number of cancer cells in the equilibrium phase undergoing Darwinian evolution. Hence, there would be less development of the immune system evading cancers using this approach to cancer prevention.
Treatment of Cancer
According to the immune suppression model of cancer, when the immune system response goes down due to aging, lifestyle, and so on, covert cancers can start growing and manifest as early-stage overt cancers. When such early-stage cancers are detected through traditional cancer screening methods or observed symptoms, a boosted immune system would likely be able to eliminate the cancers or bring them under control again, converting them back to covert cancers. Thus, a method of treating early-stage cancers would be to boost the immune system, and this can be achieved, for example, with exercise, infection, and low-dose radiation. Even for late-stage cancers, enhancing the immune system with infection has resulted in complete remission of cancer.81 Hence, immune system boosting interventions should be tested in clinical trials for all stages of cancers.
Physical activity increases immune system response,53 and studies have indicated that physical activity improves survival in patients with cancer overall82 as well as for colorectal cancer,83 breast cancer,84 and prostate cancer,85 suggesting the study of exercise for treating cancers. A recent review has indicated that structured exercise may have a potential of reducing cancers in patients with early-stage cancer.86
Another method of boosting the immune system is via infection.54 Postoperative infection in patients with osteosarcoma has been associated with improved survival87 supporting the concept that infection may be helpful in treating cancers. Spontaneous remission of cancer has been observed to be highly correlated with prior feverish infections,73 indicating that infection had a cancer therapeutic effect. The use of mixed bacteria vaccine for treating cancers was studied over a century ago by Coley88 and attained results comparable to results achieved with modern-day treatments.55 Hence, such a mixed bacteria vaccine should be studied for the treatment of cancer.89
A third method of enhancing the immune system is through exposure to low-dose radiation.61,90 Repeated application of low-dose radiation to the whole body has been utilized for treating cancer in several clinical trials and has resulted in patient survival comparable to or better than chemotherapy and so has been recommended as a method of cancer treatment.91-93 Adjuvant whole-body or half-body low-dose radiation treatments interspersed between radiation therapy sessions resulted in better patient survival compared to radiation therapy alone.93 In these studies, whole body dose of 10 or 15 cGy was given 15 or 10 times during 5 weeks, respectively, for a total dose of 1.5 Gy. Since there was concern regarding increased likelihood of leukemia when total dose of 2 Gy or more had been utilized for the treatments,94 clinical trials should be conducted with lower cumulative dose from the low-dose radiation treatments to determine if such treatments are effective in eliminating the cancers while reducing the chance for increased leukemias. The lower cumulative radiation dose may also be helpful in reducing the occurrence of thrombocytopenia sometimes observed following such treatments in the clinical trials.93,95
These and other immune system boosting treatments should be tailored to the individual to achieve the most enhancement of the immune system. For example, if the person diagnosed with early-stage cancer had led a sedentary lifestyle, a change in lifestyle that includes vigorous exercise would likely boost the immune system. If the patient had already been exercising regularly when diagnosed with cancer, a more intense exercise regimen should be prescribed to boost the immune system to higher levels than earlier. A single method of boosting the immune system would not be sufficient for eliminating the cancers. Hence, clinical trials need to be conducted to study the efficacy of applying all the above methods (and any other additional methods of boosting the immune system) simultaneously or in sequence for treating cancers. For those cancers that do not respond to the immune system boosting treatments, traditional treatment methods such as surgery, radiation therapy, chemotherapy, immunotherapy, and so on would need to be applied.
Discussion
An alternative model of cancer, the immune suppression model of cancer, has been proposed here as the basis for dealing with cancer because the present approach to cancer, which is based on the invalid somatic mutation model of cancer, has not been successful in reducing the adverse impact of cancer, despite major advances in the field during the past several decades. The recommended approach to cancer screening, of identifying individuals with weakened immune system response through assays, needs to be tested in clinical trials to ascertain its validity. Considerable evidence supports the suggested approach of boosting the immune system using exercise, infection, and low-dose radiation to prevent and treat cancer. Other interventions that increase the immune system response such as reducing obesity, smoking cessation, fruit/vegetable diet, fasting, and so on—if they are applicable—should also be studied. By applying these interventions simultaneously or in sequence, we may be able to achieve a much higher reduction in cancer mortality rates than the reductions that individual interventions have achieved in the past. The immune system boosting interventions would eliminate cancerous/precancerous cells more efficiently and so would reduce Darwinian evolution of cancers that evade the immune system. There would also be very few adverse side effects from the suggested treatments. In summary, this changed paradigm of cancer screening, prevention, and treatment based on the immune suppression model of cancer can result in a significant reduction of cancer incidence, cancer treatment adverse side effects, and cancer mortality rates, which have not been possible with the present approach that is based on the somatic mutation model of cancer.
Conclusion
Clinical trials are needed to validate the changed paradigm of cancer screening, prevention, and treatment based on the immune suppression model of cancer. Existing evidence indicates this approach would be successful in substantially reducing the adverse impact of cancer. Adoption of the proposed cancer paradigm can pay rich dividends in terms of significantly reduced cancer incidence and mortality rates while essentially eliminating the adverse side effects of present cancer treatments.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Eckhouse S, Lewison G, Sullivan R. Trends in the global funding and activity of cancer research. Mol Oncol. 2008;2(1):20–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. [DOI] [PubMed] [Google Scholar]
- 3. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. [DOI] [PubMed] [Google Scholar]
- 4. Timeline: Major Milestones Against Cancer. American Society of Clinical Oncology. 2013. http://www.cancerprogress.net/timeline/major-milestones-against-cancer. Accessed August 1, 2016.
- 5. Hunter DJ, Reddy KS. Noncommunicable diseases. N Engl J Med. 2013;369(14):1336–1343. [DOI] [PubMed] [Google Scholar]
- 6. Goldstein I, Madar S, Rotter V. Cancer research, a field on the verge of a paradigm shift? Trends Mol Med. 2012;18(6):299–303. [DOI] [PubMed] [Google Scholar]
- 7. Diamandis EP, Bast RC, Jr, Lopez-Otin C. Conquering cancer in our lifetime: new diagnostic and therapeutic trends. Clin Chem. 2013;59(1):1–3. [DOI] [PubMed] [Google Scholar]
- 8. Hanahan D. Rethinking the war on cancer. Lancet. 2014;383(9916):558–563. [DOI] [PubMed] [Google Scholar]
- 9. Soto AM, Sonnenschein C. The somatic mutation theory of cancer: growing problems with the paradigm? Bioessays. 2004;26(10):1097–1107. [DOI] [PubMed] [Google Scholar]
- 10. Tomasetti C, Vogelstein B. Cancer etiology. Variation in cancer risk among tissues can be explained by the number of stem cell divisions. Science. 2015;347(6217):78–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Etzioni R, Urban N, Ramsey S, et al. The case for early detection. Nat Rev Cancer. 2003;3(4):243–252. [DOI] [PubMed] [Google Scholar]
- 12. Smith RA, Manassaram-Baptiste D, Brooks D, et al. Cancer screening in the United States, 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65(1):30–54. [DOI] [PubMed] [Google Scholar]
- 13. Lees BF, Erickson BK, Huh WK. Cervical cancer screening: evidence behind the guidelines. Am J Obstet Gynecol. 2016;214(4):438–443. [DOI] [PubMed] [Google Scholar]
- 14. Ahn HS, Kim HJ, Welch HG. Korea’s thyroid-cancer “epidemic”—screening and overdiagnosis. N Engl J Med. 2014;371(19):1765–1767. [DOI] [PubMed] [Google Scholar]
- 15. Gotzsche PC, Jorgensen KJ. Screening for breast cancer with mammography. Cochrane Database Syst Rev. 2013(6):CD001877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fuller MS, Lee CI, Elmore JG. Breast cancer screening: an evidence-based update. Med Clin North Am. 2015;99(3):451–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kim EH, Andriole GL. Prostate-specific antigen-based screening: controversy and guidelines. BMC Med. 2015;13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Morrell S, Barratt A, Irwig L, Howard K, Biesheuvel C, Armstrong B. Estimates of overdiagnosis of invasive breast cancer associated with screening mammography. Cancer Causes Control. 2010;21(2):275–282. [DOI] [PubMed] [Google Scholar]
- 19. Welch HG, Black WC. Overdiagnosis in cancer. J Natl Cancer Inst. 2010;102(9):605–613. [DOI] [PubMed] [Google Scholar]
- 20. Moyer VA. Screening for prostate cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2012;157(2):120–134. [DOI] [PubMed] [Google Scholar]
- 21. Pettit SD, Lipshultz SE, Cleeland CS, et al. Enhancing quality of life as a goal for anticancer therapeutics. Sci Translat Med. 2016;8(344):344ed9. [DOI] [PubMed] [Google Scholar]
- 22. Luqmani YA. Breast screening: an obsessive compulsive disorder. Cancer Causes Control. 2014;25(10):1423–1426. [DOI] [PubMed] [Google Scholar]
- 23. Loeb S, Bjurlin MA, Nicholson J, et al. Overdiagnosis and overtreatment of prostate cancer. Eur Urol. 2014;65(6):1046–1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Donovan JL. Presenting treatment options to men with clinically localized prostate cancer: the acceptability of active surveillance/monitoring. J Natl Cancer Inst Monogr. 2012;2012(45):191–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Xu J, Neale AV, Dailey RK, Eggly S, Schwartz KL. Patient perspective on watchful waiting/active surveillance for localized prostate cancer. J Am Board Fam Med. 2012;25(6):763–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Prasad V, Lenzer J, Newman DH. Why cancer screening has never been shown to “save lives”—and what we can do about it. BMJ. 2016;352:h6080. [DOI] [PubMed] [Google Scholar]
- 27. Kochanek KD, Murphy SL, Xu J, Tejada-Vera B. Deaths: final data for 2014. Natl Vital Stat Rep. 2016;65(4):1–122. [PubMed] [Google Scholar]
- 28. AACR Cancer Progress Report 2012: American Association for Cancer Research. http://cancerprogressreport.org/2011/Documents/2011_AACR_CPR_Text_8-03-12.pdf. Accessed August 1, 2016. [DOI] [PubMed]
- 29. Thun MJ, Jemal A. How much of the decrease in cancer death rates in the United States is attributable to reductions in tobacco smoking? Tob Control. 2006;15(5):345–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Soto AM, Sonnenschein C. One hundred years of somatic mutation theory of carcinogenesis: is it time to switch? Bioessays. 2014;36(1):118–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Sonnenschein C, Soto AM. Somatic mutation theory of carcinogenesis: why it should be dropped and replaced. Mol Carcinog. 2000;29(4):205–211. [DOI] [PubMed] [Google Scholar]
- 32. DeGregori J. Challenging the axiom: does the occurrence of oncogenic mutations truly limit cancer development with age? Oncogene. 2013;32(15):1869–1875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Cancer Incidence by Age in the UK in 2011–2013. Cancer Research UK. http://www.cancerresearchuk.org/health-professional/cancer-statistics/incidence/age#heading-Zero. Accessed June 22, 2016.
- 34. Imaida K, Hasegawa R, Kato T, et al. Clinicopathological analysis on cancers of autopsy cases in a geriatric hospital. Pathol Int. 1997;47(5):293–300. [DOI] [PubMed] [Google Scholar]
- 35. Greaves M. Does everyone develop covert cancer? Nat Rev Cancer. 2014;14(4):209–210. [DOI] [PubMed] [Google Scholar]
- 36. Martincorena I, Roshan A, Gerstung M, et al. High burden and pervasive positive selection of somatic mutations in normal human skin. Science. 2015;348(6237):880–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bagnardi V, Rota M, Botteri E, et al. Alcohol consumption and site-specific cancer risk: a comprehensive dose–response meta-analysis. Br J Cancer. 2015;112(3):580–593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. National Toxicology Program. NTP toxicology and carcinogeneis studies of sodium azide (CAS: 26628-22-8) in F344 rats (gavage studies). Natl Toxicol Program Tech Rep Ser. 1991;389:1–165. [PubMed] [Google Scholar]
- 39. Maciak S, Michalak P. Cell size and cancer: a new solution to Peto’s paradox? Evol Appl. 2015;8(1):2–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Haas D, Ablin AR, Miller C, Zoger S, Matthay KK. Complete pathologic maturation and regression of stage IVS neuroblastoma without treatment. Cancer. 1988;62(4):818–825. [DOI] [PubMed] [Google Scholar]
- 41. Fogarty MC, Hughes CM, Burke G, et al. Exercise-induced lipid peroxidation: implications for deoxyribonucleic acid damage and systemic free radical generation. Environ Mol Mutagen. 2011;52(1):35–42. [DOI] [PubMed] [Google Scholar]
- 42. Orsini N, Mantzoros CS, Wolk A. Association of physical activity with cancer incidence, mortality, and survival: a population-based study of men. Br J Cancer. 2008;98(11):1864–1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Oliveira Cobucci RN, Saconato H, Lima PH, et al. Comparative incidence of cancer in HIV-AIDS patients and transplant recipients. Cancer Epidemiol. 2012;36(2):e69–e73. [DOI] [PubMed] [Google Scholar]
- 44. Acuna SA, Fernandes KA, Daly C, et al. Cancer mortality among recipients of solid-organ transplantation in Ontario, Canada. JAMA Oncol. 2016;2(4):463–469. [DOI] [PubMed] [Google Scholar]
- 45. Koebel CM, Vermi W, Swann JB, et al. Adaptive immunity maintains occult cancer in an equilibrium state. Nature. 2007;450(7171):903–907. [DOI] [PubMed] [Google Scholar]
- 46. Levin MJ. Immune senescence and vaccines to prevent herpes zoster in older persons. Curr Opin Immunol. 2012;24(4):494–500. [DOI] [PubMed] [Google Scholar]
- 47. Corthay A. Does the immune system naturally protect against cancer? Front Immunol. 2014;5(197):1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Furman D, Hejblum BP, Simon N, et al. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proc Natl Acad Sci U S A. 2014;111(2):869–874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2015. CA Cancer J Clin. 2015;65(1):5–29. [DOI] [PubMed] [Google Scholar]
- 50. Wang H, Diepgen TL. Is atopy a protective or a risk factor for cancer? A review of epidemiological studies. Allergy. 2005;60(9):1098–111. [DOI] [PubMed] [Google Scholar]
- 51. Turfkruyer M, Verhasselt V. Breast milk and its impact on maturation of the neonatal immune system. Curr Opin Infect Dis. 2015;28(3):199–206. [DOI] [PubMed] [Google Scholar]
- 52. Amitay EL, Keinan-Boker L. Breastfeeding and childhood leukemia incidence: a meta-analysis and systematic review. JAMA Pediatr. 2015;169(6):e151025. [DOI] [PubMed] [Google Scholar]
- 53. Woods JA, Keylock KT, Lowder T, et al. C exercise training extends influenza vaccine seroprotection in sedentary older adults: the immune function intervention trial. J Am Geriatr Soc. 2009;57(12):2183–2191. [DOI] [PubMed] [Google Scholar]
- 54. Karbach J, Neumann A, Brand K, et al. Phase I clinical trial of mixed bacterial vaccine (Coley’s toxins) in patients with NY-ESO-1 expressing cancers: immunological effects and clinical activity. Clin Cancer Res. 2012;18(19):5449–5459. [DOI] [PubMed] [Google Scholar]
- 55. Richardson MA, Ramirez T, Russell NC, Moye LA. Coley toxins immunotherapy: a retrospective review. Altern Ther Health Med. 1999;5(3):42–47. [PubMed] [Google Scholar]
- 56. Liu SZ. Nonlinear dose–response relationship in the immune system following exposure to ionizing radiation: mechanisms and implications. Nonlinearity Biol Toxicol Med. 2003;1(1):71–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Stampfli MR, Anderson GP. How cigarette smoke skews immune responses to promote infection, lung disease and cancer. Nat Rev Immunol. 2009;9(5):377–384. [DOI] [PubMed] [Google Scholar]
- 58. Molina PE, Happel KI, Zhang P, Kolls JK, Nelson S. Focus on: alcohol and the immune system. Alcohol Res Health. 2010;33(1-2):97–108. [PMC free article] [PubMed] [Google Scholar]
- 59. Ozasa K, Shimizu Y, Suyama A, et al. Studies of the mortality of atomic bomb survivors, report 14, 1950–2003: an overview of cancer and noncancer diseases. Radiat Res. 2012;177(3):229–243. [DOI] [PubMed] [Google Scholar]
- 60. Nelson DE, Jarman DW, Rehm J, et al. Alcohol-attributable cancer deaths and years of potential life lost in the United States. Am J Public Health. 2013;103(4):641–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yang G, Kong Q, Wang G, et al. Low-dose ionizing radiation induces direct activation of natural killer cells and provides a novel approach for adoptive cellular immunotherapy. Cancer biother radiopharm. 2014;29(10):428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Hwang SL, Guo HR, Hsieh WA, et al. Cancer risks in a population with prolonged low dose-rate gamma-radiation exposure in radiocontaminated buildings, 1983–2002. Int J Radiat Biol. 2006;82(12):849–858. [DOI] [PubMed] [Google Scholar]
- 63. Sponsler R, Cameron JR. Nuclear shipyard worker study 1980–1988: a large cohort exposed to low-dose-rate gamma radiation. Int J Low Radiat. 2005;1(4):463–478. [Google Scholar]
- 64. Berrington A, Darby SC, Weiss HA, Doll R. 100 years of observation on British radiologists: mortality from cancer and other causes 1897–1997. Br J Radiol. 2001;74(882):507–519. [DOI] [PubMed] [Google Scholar]
- 65. Kostyuchenko VA, Krestinina L. Long-term irradiation effects in the population evacuated from the East-Urals radioactive trace area. Sci Total Environ. 1994;142(1-2):119–125. [DOI] [PubMed] [Google Scholar]
- 66. Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. [DOI] [PubMed] [Google Scholar]
- 67. Vesely MD, Kershaw MH, Schreiber RD, Smyth MJ. Natural innate and adaptive immunity to cancer. Annu Rev Immunol. 2011;29:235–271. [DOI] [PubMed] [Google Scholar]
- 68. Jyonouchi H, Sun S, Le H. Proinflammatory and regulatory cytokine production associated with innate and adaptive immune responses in children with autism spectrum disorders and developmental regression. J Neuroimmunol. 2001;120(1-2):170–179. [DOI] [PubMed] [Google Scholar]
- 69. Haskell WL, Lee IM, Pate RR, et al. Physical activity and public health: updated recommendation for adults from the American College of Sports Medicine and the American Heart Association. Circulation. 2007;116(9):1081–1093. [DOI] [PubMed] [Google Scholar]
- 70. Centers for Disease Control and Prevention (CDC). Adult participation in aerobic and muscle-strengthening physical activities—United States, 2011. MMWR Morb Mortal Wkly Rep. 2013;62(17):326–330. [PMC free article] [PubMed] [Google Scholar]
- 71. Hallal PC, Andersen LB, Bull FC, Guthold R, Haskell W, Ekelund U; Lancet Physical Activity Series Working Group. Global physical activity levels: surveillance progress, pitfalls, and prospects. Lancet. 2012;380(9838):247–257. [DOI] [PubMed] [Google Scholar]
- 72. Rudant J, Lightfoot T, Urayama KY, et al. Childhood acute lymphoblastic leukemia and indicators of early immune stimulation: a childhood leukemia international consortium study. Am J Epidemiol. 2015;181(8):549–562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Kleef R, Jonas WB, Knogler W, Stenzinger W. Fever, cancer incidence and spontaneous remissions. Neuroimmunomodulation. 2001;9(2):55–64. [DOI] [PubMed] [Google Scholar]
- 74. Feinendegen LE, Pollycove M, Neumann RD. Hormesis by low dose radiation effects: low-dose cancer risk modeling must recognize up-regulation of protection In: Baum RP, ed. Therapeutic Nuclear Medicine. Heidelberg: Springer; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Koana T, Tsujimura H. A U-shaped dose–response relationship between x radiation and sex-linked recessive lethal mutation in male germ cells of Drosophila. Radiat Res. 2010;174(1):46–51. [DOI] [PubMed] [Google Scholar]
- 76. Osipov AN, Buleeva G, Arkhangelskaya E, Klokov D. In vivo gamma-irradiation low dose threshold for suppression of DNA double strand breaks below the spontaneous level in mouse blood and spleen cells. Mutat Res. 2013;756(1-2):141–145. [DOI] [PubMed] [Google Scholar]
- 77. Gasser S, Raulet DH. The DNA damage response arouses the immune system. Cancer Res. 2006;66(8):3959–3962. [DOI] [PubMed] [Google Scholar]
- 78. de Heredia FP, Gomez-Martinez S, Marcos A. Obesity, inflammation and the immune system. Proc Nutr Soc. 2012;71(2):332–338. [DOI] [PubMed] [Google Scholar]
- 79. Maijo M, Clements SJ, Ivory K, Nicoletti C, Carding SR. Nutrition, diet and immunosenescence. Mech Ageing Dev. 2014;136-137:116–128. [DOI] [PubMed] [Google Scholar]
- 80. Cheng CW, Adams GB, Perin L, et al. Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression. Cell Stem Cell. 2014;14(6):810–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Jessy T. Immunity over inability: the spontaneous regression of cancer. J Nat Sci Biol Med. 2011;2(1):43–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Lee IM, Wolin KY, Freeman SE, Sattlemair J, Sesso HD. Physical activity and survival after cancer diagnosis in men. J Phys Act Health. 2014;11(1):85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Meyerhardt JA, Giovannucci EL, Holmes MD, et al. Physical activity and survival after colorectal cancer diagnosis. J Clin Oncol. 2006;24(22):3527–3534. [DOI] [PubMed] [Google Scholar]
- 84. Ibrahim EM, Al-Homaidh A. Physical activity and survival after breast cancer diagnosis: meta-analysis of published studies. Med Oncol. 2011;28(3):753–765. [DOI] [PubMed] [Google Scholar]
- 85. Friedenreich CM, Wang Q, Neilson HK, Kopciuk KA, McGregor SE, Courneya KS. Physical activity and survival after prostate cancer [published online January 7, 2016]. Eur Urol. pii: S0302-2838(15)01241-5. [DOI] [PubMed] [Google Scholar]
- 86. Scott JM, Koelwyn GJ, Hornsby WE, et al. Exercise therapy as treatment for cardiovascular and oncologic disease after a diagnosis of early-stage cancer. Semin Oncol. 2013;40(2):218–228. [DOI] [PubMed] [Google Scholar]
- 87. Jeys LM, Grimer RJ, Carter SR, Tillman RM, Abudu A. Post operative infection and increased survival in osteosarcoma patients: are they associated? Ann Surg Oncol. 2007;14(10):2887–2895. [DOI] [PubMed] [Google Scholar]
- 88. Bickels J, Kollender Y, Merinsky O, Meller I. Coley’s toxin: historial perspective. Isr Med Assoc J. 2002;4(6):471–472. [PubMed] [Google Scholar]
- 89. Wong S, Slavcev RA. Treating cancer with infection: a review on bacterial cancer therapy. Lett Appl Microbiol. 2015;61(2):107–112. [DOI] [PubMed] [Google Scholar]
- 90. Yang G, Li W, Jiang H, et al. Low-dose radiation may be a novel approach to enhance the effectiveness of cancer therapeutics [published online November 15, 2016]. Int J Cancer. 139(10):2157–2168. [DOI] [PubMed] [Google Scholar]
- 91. Cuttler JM, Pollycove M. Can cancer be treated with low doses of radiation? J Am Phys Surg. 2003;8(4):108–111. [Google Scholar]
- 92. Pollycove M. Radiobiological basis of low-dose irradiation in prevention and therapy of cancer. Dose Response. 2007;5(1):26–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Sakamoto K. Radiobiological basis for cancer therapy by total or half-body irradiation. Nonlinearity Biol Toxicol Med. 2004;2(4):293–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Travis LB, Weeks J, Curtis RE, et al. Leukemia following low-dose total body irradiation and chemotherapy for non-Hodgkin’s lymphoma. J Clin Oncol. 1996;14(2):565–571. [DOI] [PubMed] [Google Scholar]
- 95. Chaffey JT, Rosenthal DS, Moloney WC, Hellman S. Total body irradiation as treatment for lymphosarcoma. Int J Radiat Oncol Biol Phys. 1976;1(5-6):399–405. [DOI] [PubMed] [Google Scholar]