Table 1.
| Entry | Quinazolinone | Product | Yieldc | e.r.d |
|---|---|---|---|---|
| 1 | 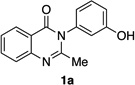 |
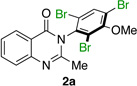 |
86% | 97:3 |
| 2 | 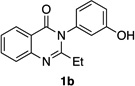 |
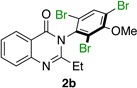 |
79% | 97:3 |
| 3 | 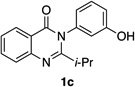 |
 |
78% | 96:4 |
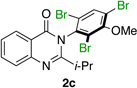
| 4 |  |
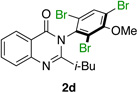 |
79% | 96:4 |
| 5 | 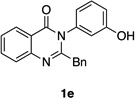 |
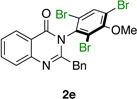 |
75% | 93:7 |
| 6 | 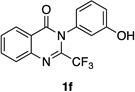 |
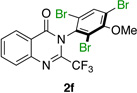 |
63% | 63:37 |
| 7 | 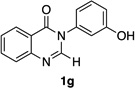 |
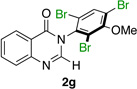 |
80% | 65:35 |
| 8 | 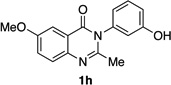 |
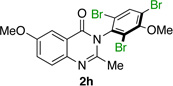 |
85% | 95:5 |
| 9 | 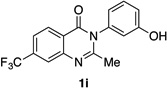 |
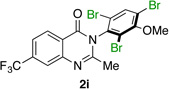 |
85% | 93:7 |
| 10e | 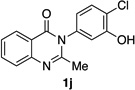 |
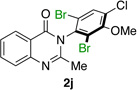 |
93% | 96:4 |
| 11e | 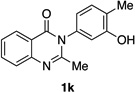 |
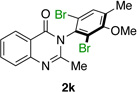 |
92% | 99:1 |
| 12 | 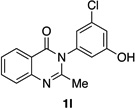 |
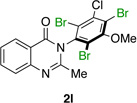 |
89% | 96:4 |
| 13 | 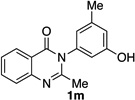 |
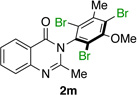 |
77% | 98:2 |
| 14e | 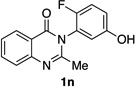 |
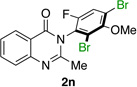 |
84% | 56:44 |
Reaction Conditions: quinazolinone 1 (0.10 mmol, 1 equiv), peptide 4q (0.01 mmol, 10 mol% w.r.t. 1), NBS (0.30 mmol, 3 equiv w.r.t. 1), PhMe/CHCl3 (9:1 v/v) with 5% acetone additive (by volume), slow addition of NBS over 2.5 h.
Data represent the average of two trials.
Isolated yields after chromatography are presented.
Enantiomer ratios were determined by chiral HPLC using OJ-H or AD-H columns.
2.0 equiv NBS was used in the bromination.
