Abstract
The current study is conducted to investigate efficacy of the chemotherapy drug paclitaxel in combination with Avastin (Roche Diagnostics GmbH., Mannheim, Germany) (antiangiogenic agent) in treatment of malignant pleural effusions (MPEs).
Twenty-four patients with non–small cell lung cancer were randomly assigned for 2 treatment approaches. Ten patients received paclitaxel (175 mg/m2) alone, and 14 patients took a combination therapy of paclitaxel and Avastin (5 mg/kg). Efficacy of the treatment approaches in the patients was validated with the change in the MPE volume. Pharmacokinetic (PK) profile and urinary excretion rate of paclitaxel were analyzed with serum vascular endothelial growth factor (VEGF) level, and adverse events were examined as well.
The combination therapy reduced the MPE level with a successful rate of 29% and a survival rate of 25% over the single paclitaxel treatment in the study cohort (both P < 0.05). PKs for the combined treatment displayed a rapid distribution of the anticancer drug paclitaxel with an obvious increase in its elimination half-life in the pleural fluid (both P < 0.01). Mean residence time of paclitaxel increased in the presence of Avastin (P < 0.01). Serum VEGF levels significantly reduced in the Avastin-treated patients as compared to the paclitaxel-treated ones (P < 0.01). The urinary excretion rate was similar in the study cohort. Incidence of adverse events for the 2 treatment approaches was similar in the patients.
Intervention of Avastin enhances potency of paclitaxel in treatment of MPEs with the increased survival rate of the patients through inhibiting VEGF production and prolonging time of ongoing interaction between the chemotherapy drug and the tumor tissues.
Keywords: Avastin, malignant pleural effusion, NSCLC, paclitaxel, pharmacokinetics, VEGF
1. Introduction
Malignant pleural effusion (MPEs) are a common clinical problem in patients with primary thoracic malignancy and metastatic malignancy to the thorax.[1–3] Median survival following diagnosis ranges from 3 to 12 months and is dependent on the stage and type of the underlying malignancy.[4] Since the presence of MPEs generally indicates that the malignancy cannot be cured by surgery, the goal of treatment for MPEs is to relieve the symptom of breathlessness.[5] For this reason, careful consideration of the patient's expected survival and quality of life is needed when deciding the optimum treatment modality in such patients. Options for management of MPEs mainly include repeated therapeutic thoracentesis, indwelling pleural catheter (IPC), and chemical pleurodesis.[6]
Avastin (Roche Diagnostics GmbH., Mannheim, Germany) is a monoclonal antibody that blocks angiogenesis by inhibiting vascular endothelial growth factor (VEGF).[7] Avastin works as a tumor-starving (antiangiogenic) therapy to control the growth of new blood vessels around and inside of tumor tissues.[8] VEGF, also known as vascular permeability factor, is a potent inducer of capillary permeability and has been demonstrated at a high level in the exudative pleural effusions.[9] VEGF is believed to function as a tumor angiogenesis factor being mitogenic for endothelial cells and has a potential pathogenic role in the development of pleural effusions.[10] In this era of targeted therapies for MPEs, inhibiting angiogenesis seems logically considerable.
In this study, efficacy of the chemotherapy drug paclitaxel in combination with Avastin in treatment of MPEs was examined in the non–small cell lung cancer (NSCLC) patients based on the consideration that Avastin is an angiogenesis inhibitor that may reduce the growth of new blood vessels through inhibiting the VEGF production.[11] Our result indicated that intervention of Avastin may obviously improve efficacy of the anticancer drug paclitaxel in treatment of MPEs through inhibiting VEGF production and increasing the time length of the drug remaining within the diseased tissues.
2. Patients and methods
2.1. Patients and inclusion criteria
We recruited 24 NSCLC patients required for treatment of MPEs in the period from January 2013 to September 2015. Clinical characteristics of these patients and the results of statistical analysis are shown in Table 1. Major inclusion criteria selected for this study were all patients histopathologically diagnosed with adenocarcinoma at the stages of IV-M1a or IV-M1b according to the International Association for the Study of Lung Cancer[12]; Karnofsky Performance Status ≥60[13]; MPE demonstrated by the identification of malignant cells in pleural fluid due to metastases originating from the tumors in the lung; pleural fluid volume estimated by using a combination method of chest radiography, computer technology (CT) scan, and B-ultrasound scan of chest; no abnormal findings on electrocardiography, bone marrow, liver and kidney function tests; no allergic reaction to 2 chemotherapy drugs—paclitaxel and Avastin.
Table 1.
Clinical characteristics of the investigated patients.
The study has been approved by the Ethics Committee of the First Affiliated Hospital, Chinese PLA General Hospital; the Ethics Committee also approved the related screening, treatment, and data collection from these patients based on the experimental design and analysis of clinical outcome. The methods were carried out in accordance with the approved guidelines and performed according to the Declaration of Helsinki. All subjects signed written informed consent forms for this study. This statement explicitly states that “informed” consent was obtained from subjects’ families.
2.2. Therapeutic approach
The subjects were randomly assigned to individual treatments by 2 treatment methods. Oral dexamethasone (20 mg), intramuscular diphenhydramine (40 mg), and intravenous omeprazole (40 mg) were given before treatments of paclitaxel (Gorden Pharma Latina S.P.A. Sermoneta, Italy) and Avastin. Ten patients slowly received intrapleural infusion of paclitaxel (175 mg/m2) dissolved in 500 mL of 0.9% saline solution, and 14 patients were treated with the same dose of paclitaxel after an intrapleural injection of Avastin (5 mg/kg) in 100 mL of the solution once every 3 weeks for 12 consecutive weeks. To ensure equal distribution of the drugs in the pleural cavity, a body position of the patients required to be turned over at an interval of 15 minutes within an initial 2 hours after the treatments.
The pigtail catheter (Suzhou Jingxin Medical Supplies Co., Ltd., Suzhou, China) was applied to the patients with MPEs for chest drainage and infusion of drugs. All procedures were done at the bedside with the B-ultrasound guidance. Salient technical aspects of pigtail catheter insertion included appropriate use of local anesthetic and needle insertion to avoid the intercostal bundle. We typically employed a small needle (16 ga) before inserting the larger one provided with the kit. In this way, pleural fluid was easily withdrawn with the needle, and passage of the guidewire into the pleural space would be virtually effortless. There are 6 side holes at the distal end of the catheter. With an adequate insertion of the pigtail, the sideholes were well within the pleural cavity for proper function. The pigtail catheter was attached to a standard thoracic drainage system, and suction was applied for treatment of pleural fluid. Furthermore, IPC may benefit intervention of current drugs.
2.3. Observation of clinical efficacy and survival rate
Efficacy of the drugs in treatment of MPEs was assessed according to clinical manifestations and imaging techniques of chest radiographs and CT scan made by American Thoracic Society.[3] The standards of drug efficacy evaluation include complete relief (CR) and absence of pleural effusions with symptom of dyspnea significantly improved; incomplete relief (IR), <50% of an initial volume of MPE with the degree of dyspnea alleviated; treatment failure, MPE level >50% of the initial finding or fluid drainage required within 2 months following the initiation of therapy; overall efficacy (OE) defined as CR + IR in treatment of MPE.
Survival rates of the treated patients were examined with 1-year follow-up after treatment. The numbers of survivors were expressed as a percentage of population proportion in each group of the patients.
2.4. Analyses of pharmacokinetics
Since a drug's bioavailability can be defined as the proportion of the drug that reaches its site of action, pharmacokinetic (PK) modeling is performed by a noncompartmental method which may estimate the exposure to a drug by calculating the area under the curve of a concentration–time graph. Maximum observed concentration (Cmax), a half-life (T1/2α) for apparent volume of drug distribution, elimination half-life (T1/2β), and mean residence time (MRT) for the paclitaxel treatment were analyzed. Alpha phase: an initial phase of rapid decrease in the fluid concentration. The decrease is primarily attributed to drug distribution from the pleural fluid into the target tissues. Beta phase: a phase of gradual decrease in the drug concentration in the fluid after the alpha phase. The decrease is primarily attributed to drug metabolism and excretion. The calculations of PK parameters were performed by using the software DAS 2.0 (Mathematical Pharmacological Professional Committee of China, Beijing, China).
2.5. Observation of adverse events
An adverse event is any unfavorable and unintended sign (including an abnormal laboratory finding), symptom, or disease temporally associated with the use of a medical treatment. The event is a term that is a unique representation of a specific event used for medical documentation and scientific analyses. Side effects of the drugs in treatment of MPE were classified according to Common Terminology Criteria for Adverse Events (CTCAE) v3.0. The CTCAE v3.0 may display grades 1 through 5 with unique clinical descriptions of severity for each event based on this general guideline of 1—mild, 2—moderate, 3—severe, 4—life-threatening or disabling, and 5—death related to the events.
2.6. Collection of samples and analysis of high-performance liquid chromatography
Following treatments with paclitaxel in combination with and without Avastin, 3.0 mL of pleural effusion fluid was collected at different time points of 0.5, 1, 2, 4, 6, 8, 12, 24.0, 48.0, and 72 hours, respectively. Urinary specimens were obtained at the time point of 24 hours after treatment. The supernatants of the samples were prepared with centrifuge (3000 r/min × 5 minutes) at 4 °C for the PK analysis of drug concentration. All samples were placed in sealed tubes and stored at −20 °C until use.
High-performance liquid chromatography (HPLC) was used to separate, identify, and quantify drug paclitaxel level in the collected specimens. The preparation of sample mixtures was finished by following the manufacturing introduction (Dikma Co, Beijing, China). Briefly, a Dikma Diamonsil C18 analytical column (4.6 mm ID × 150 mm length for a particle size of 5 μm) was applied to measure paclitaxel level in the pleural fluid and urine samples. The sample was dissolved in the eluent containing methanol, acetonitrile, and water (40:34:26, v/v/v), and then it was injected into HPLC system about 50 μL. The component of drug paclitaxel was isocratically eluted at a flow rate of 1 mL/min at 30 °C. All data were analyzed at a wavelength of 228 nm.
2.7. Measurement of serum VEGF level
Peripheral venous blood samples were drawn into sterile glass tubes. Blood samples were allowed to coagulate at room temperature for 30 minutes and centrifuged at 2000 × g for 10 minutes. Serum VEGF levels were determined according to the manufacturer's directions. Briefly, this assay employed the quantitative sandwich enzyme immunoassay technique with monoclonal antibodies, specific for VEGF precoated on to a microplate. Standard controls and samples were pipetted into the wells in duplicate. After growth factor binding and washing, an enzyme-linked antibody specific for VEGF was added to each well. Optical density was measured at 450 nm using a microtiter plate reader (MR 5000, Dynatech Laboratories, Chantilly, VA).
2.8. Statistical analyses
Values were expressed as a percentage of distribution of the data from the investigated patients and mean ± standard error on some of the results, respectively. Statistical analysis was performed using Statistical Package for the Social Science (SPSS, version 13.0, SPSS Inc., Chicago, IL, USA). Comparisons from groups with individual measurements were performed by Student paired t test in between 2 groups. The chi-square test (χ2) was conducted to analyze the significance of a parameter within groups. A P value <0.05 was considered significant.
3. Results
3.1. Efficacy of paclitaxel plus avastin in treatment of pleural effusion
Twenty-four NSCLC patients with MPEs received intrapleural infusion of paclitaxel in presence and absence of Avastin. The pleural fluid level and the degree of dyspnea were used to validate effects of the drugs on the patients. OE and survival rates were expressed with a change of percentage in each treatment approach, and the results are shown in Fig. 1. A combination therapy of paclitaxel and Avastin significantly reduced the pleural fluid level and alleviated the symptom of dyspnea with an OE rate of 78.6% in the treated patients (Fig. 1A). In contrast, only 50% patients in the paclitaxel-treated cohort displayed the rate. Clinical efficacy of the combination therapy was more potent than paclitaxel used alone with a 29% increase in the rate in the investigated population. In terms of survival rates of 1-year follow-up (Fig. 1B), population proportion of survivors was larger in the Avastin-treated patients (45.8%) than in the paclitaxel-treated patients (20.8%). There were statistical differences in these observations between the treatments with and without Avastin (χ2 test, both P < 0.05).
Figure 1.
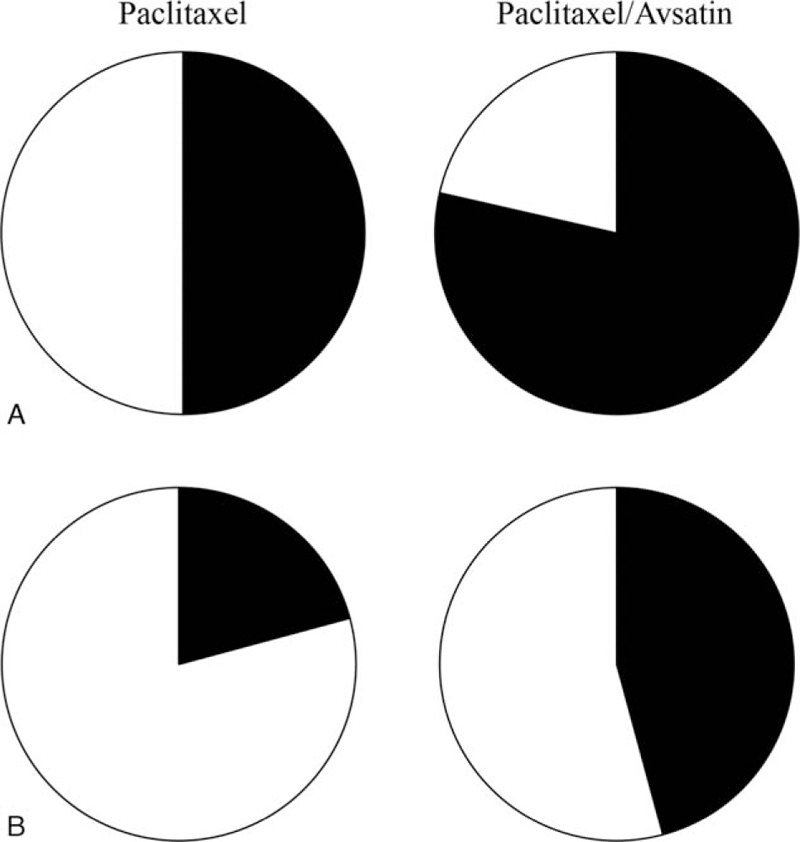
Efficacy of combination therapy and survival rate. Efficacy (A) of paclitaxel in treatment of malignant pleural effusion and survival rate (B) of the treated patients were examined over time in presence (n = 14) and absence (n = 10) of Avastin. Population distribution was marked in black (effective treatment and survivors) and white (failed treatment and patients’ death). The data were expressed as a percentage of a population proportion in the study cohort. A χ2 test for treatment efficacy and survival rates showed a P value <0.05 between the patients treated with and without Avastin.
3.2. Distribution pattern of time concentration of paclitaxel in pleural fluid
A drug's effect is often related to its concentration at the site of action, so it would be useful to monitor this concentration–effect relationship. Contents of paclitaxel in pleural fluid were determined in presence and absence of Avastin. Actual values for the changes in the pleural concentrations of paclitaxel were plotted against specific time points within an entire observation of 72 hours. The profile regarding the concentration–time curves of paclitaxel in combination with and without intervention of Avastin is shown in Fig. 2. Though both concentration–time curves declined with prolonging time courses, the curve of paclitaxel with Avastin sharply decreased as compared to that without the use of Avastin. In further analysis, the concentration–time curve for paclitaxel plus Avastin applied to the pleural fluid instantly dropped down at initial time points of 0.5 to 2 hours and then showed a gradual fall during the period of 2 to 24 hours. The curve for the combination therapy extended across to the curve of single paclitaxel treatment at the time of about 40 hours and moved up over the paclitaxel-related curve in the time period of 40 to 72 hours.
Figure 2.
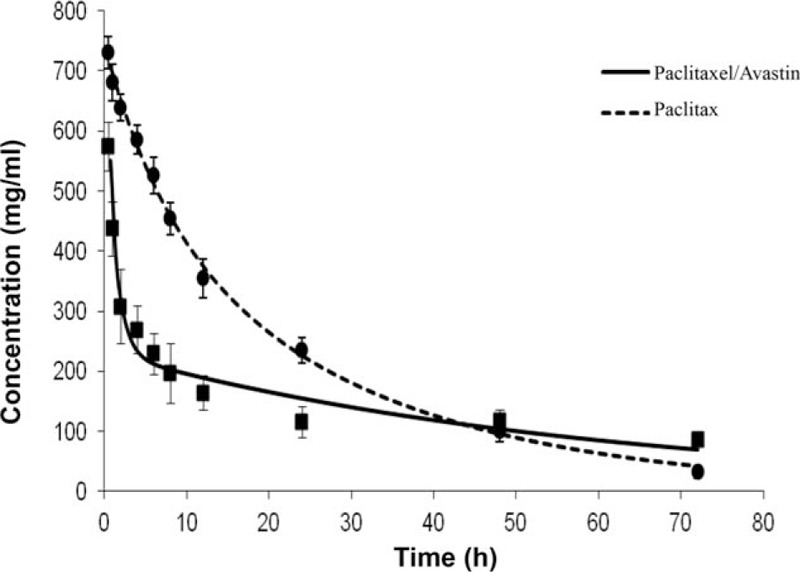
Observation on concentration–time curve of paclitaxel in pleural fluid. A concentration–time profile for the paclitaxel treatment was examined in presence (n = 14) and absence (n = 10) of Avastin. Concentration–time curves for the treatment were performed during a time period of 0 to 72 hours, and the results were expressed as the mean ± SD.
3.3. Pharmacokinetic analysis of paclitaxel in pleural fluid
Individual PK parameters for the paclitaxel treatment were determined at the time point of 72 hours in presence (n = 14) and absence (n = 10) of Avastin. The results are shown in Fig. 3. Cmax (μg/mL) for paclitaxel exhibited a similar change in the pleural fluid samples in presence and absence of Avastin. The average values were 735.82 ± 10.95 and 703.72 ± 14.15 in the paclitaxel- and the paclitaxel/Avastin-treated patients. There was a no significant difference in the Cmax value between these 2 treatment approaches. T1/2α (hour) of paclitaxel in the fluid samples was measured for their volume of distribution, and the average values were shown as 4.58 ± 0.45 and 0.83 ± 0.05 in the paclitaxel- and the paclitaxel/Avastin-treated patients. There was almost a 5.5-fold decrease in the value of T1/2α with a statistical difference seen between the treatments with and without Avastin (P < 0.01). T1/2β (hour) of paclitaxel was determined for its elimination from the pleural fluid, and the results displayed a 3-fold increase in the paclitaxel/Avastin-treated patients as compared to the paclitaxel-treated ones. The average values for the treatments were 17.41 ± 1.12 and 51.35 ± 3.67 in the paclitaxel- and the paclitaxel/Avastin-treated patients, respectively. There was a significant difference in the measurements between these 2 treatment approaches (P < 0.01). Following the intrapleural administration of the drugs, MRT (hour) of paclitaxel was calculated in the fluid, and the average values were manifested as 27.08 ± 1.12 and 65.78 ± 4.90 in the paclitaxel- and the paclitaxel/Avastin-treated patients. In contrast, there was a 2.4-fold increase in the value of MRT in the paclitaxel/Avastin-treated patients as compared to that in the paclitaxel-treated patients. There was a statistical difference in the observation between these 2 treatments (P < 0.01).
Figure 3.
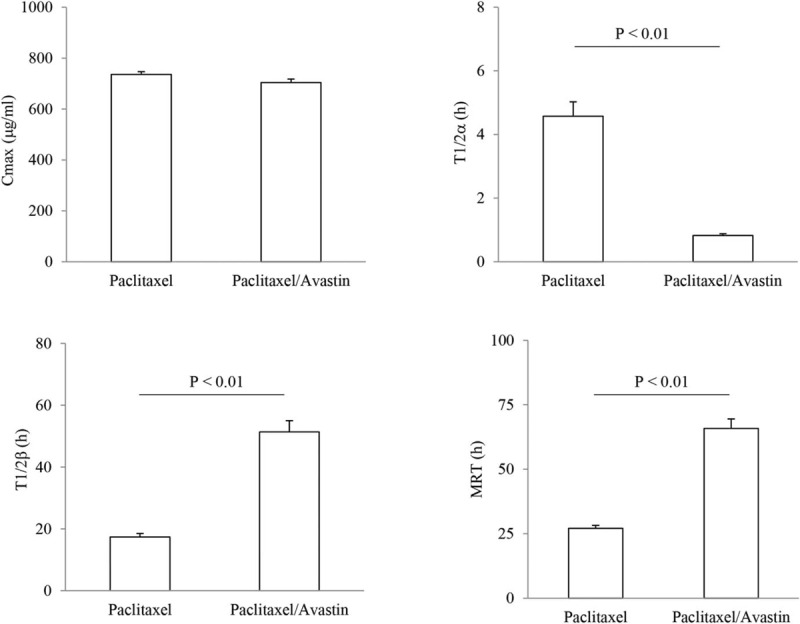
Pharmacokinetic analysis of paclitaxel. Pharmacokinetic parameters for the paclitaxel treatment were examined in presence (n = 14) and absence (n = 10) of Avastin. Paclitaxel contents in the pleural fluid were observed within the time course of 72 hours. Individual measurements for Cmax, T1/2α, T1/2β, and mean residence time were expressed as the mean ± SD.
3.4. Changes in serum VEGF level
Serum VEGF levels before and after the 2 treatment approaches were determined in presence (n = 14) and absence (n = 10) of Avastin. The results are shown in Fig. 4. Average values for VEGF concentrations (pg/mL) before and after the treatment were 124 ± 4.9 and 119.8 ± 6.9 in the paclitaxel-treated patients and 132 ± 5.8 and 64.3 ± 3.3 in the Avastin-treated ones, respectively. There were statistical differences in these measurements between either before and after treatment of Avastin or after the 2 treatment approaches (both P < 0.01).
Figure 4.
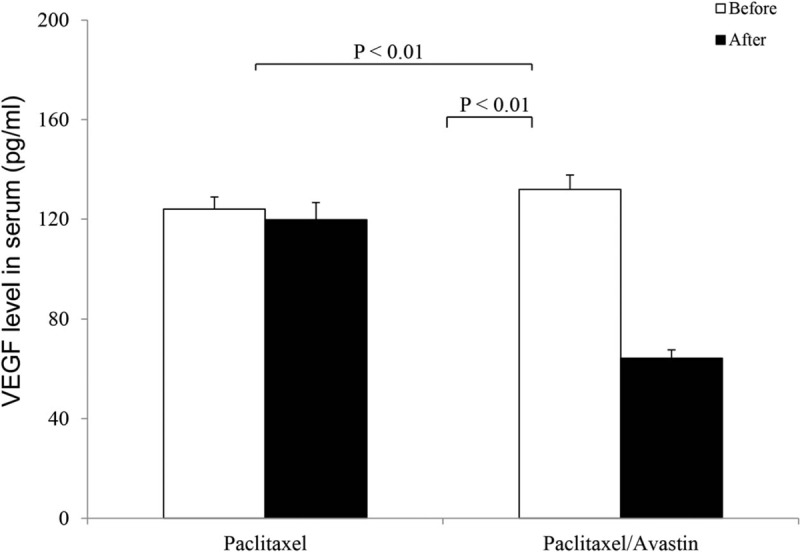
Serum vascular endothelial growth factor (VEGF) level. Serum VEGF levels before and after anticancer therapy were determined in presence (n = 14) and absence (n = 10) of Avastin. The results were expressed as the mean ± SD.
3.5. Excretion rate of paclitaxel in urine
Urinary excretion rates for the paclitaxel treatment were examined in presence (n = 14) and absence (n = 10) of Avastin, and the results are shown in Fig. 5. An excretion test was performed on 24-hour urine collections, and a similar change in the urinary excretion rate was detected in the collected specimens from the patients treated with and without Avastin. The average values (%) for the excretion rates of the drug were shown as 22% and 15% in the paclitaxel- and the paclitaxel/Avastin-treated patients, respectively. In contrast, there was no significant difference in the excretion rates between these 2 treatment approaches.
Figure 5.
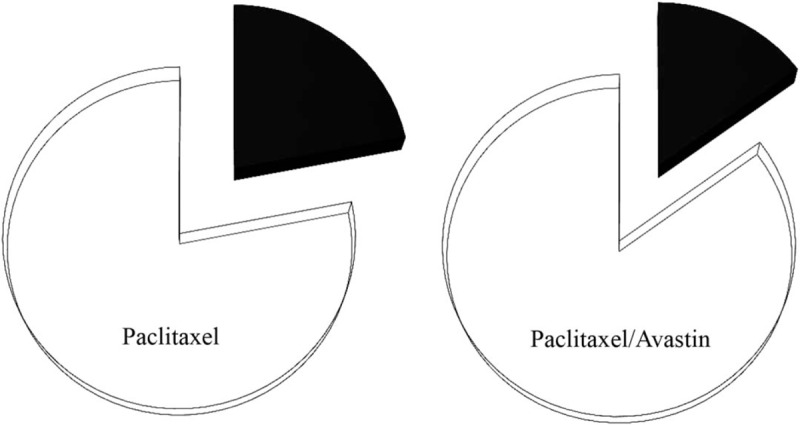
Urinary excretion rate of paclitaxel. A 24-hour urinary excretion rate for the drug paclitaxel was performed in presence (n = 14) and absence (n = 10) of Avastin. The results regarding the excretion rate of the drug (black) and the drug remained in body (white) were expressed as a percentage of the drug eliminated from the study cohort. A χ2 test showed a P value >0.05 between these 2 treatment approaches.
3.6. Analysis of adverse events
Adverse events for the paclitaxel treatment were examined in presence (n = 14) and absence (n = 10) of Avastin according to CTCAE v3.0, which is a descriptive terminology utilized for adverse event reporting. Data are calculated with a percentage of a population proportion for each treatment response, and the results are shown in Fig. 6. The most common adverse events included chest pain, low blood counts (red white cells and platelets), nausea/vomiting, diarrhea, and bleeding during the period of treatment. Severity of the side effects generally presented in a range of the grades 1 to 2. In contrast, the symptoms of low white blood cells, nausea/vomiting, and chest pain constituted a relatively large proportion in the study cohort with 50%, 38%, and 37% in the paclitaxel-treated patients and 50%, 50%, and 20% in the paclitaxel/Avastin-treated ones, respectively. However, incidence (%) of the adverse effects was not obviously different in the patients who experienced the 2 treatment methods.
Figure 6.
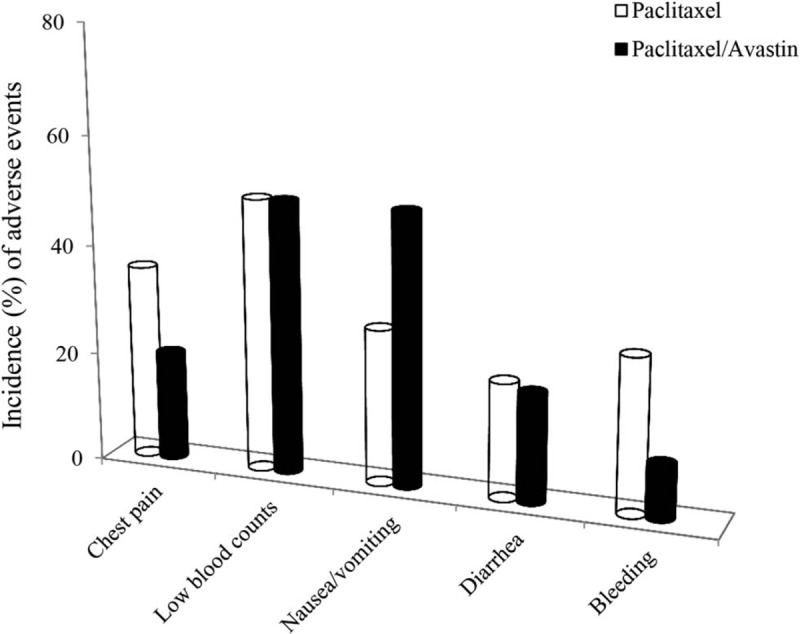
Adverse events by 2 treatment approaches. Side effects for the paclitaxel treatment were observed in presence (n = 14) and absence (n = 10) of Avastin. Most common adverse events for each treatment response are expressed with a percentage of the positive population proportion in the study cohort. A χ2 test showed a P value >0.05 between the 2 treatment approaches.
4. Discussion
To explore optimization therapy initiatives of chemotherapy drugs in management of MPEs, an antineoplastic agent in combination with Avastin was applied for the NSCLC patients in this retrospective study. The combination therapy of paclitaxel and Avastin resulted in significantly reducing pleural fluid volume with a success rate of 29% over the single treatment of paclitaxel in the investigated cohort. In addition, the numbers of survivors showed an obvious increase by 25% in the Avastin-treated patients over the paclitaxel-treated ones. These results indicated that intervention of Avastin as an inhibitor for VEGF produced an additive effect in treatment of MPEs, and the dose selected for Avastin-mediated interference with VEGF signaling was sufficient. Moreover, therapeutic thoracentesis with the IPC was feasible as a procedure for administration of consecutive drugs to reduce the presence of MPEs. Since the lung cancer especially associated with MPEs indicates advanced stage disease,[14] MPEs cause debilitating breathlessness that requires palliation by nonsurgical treatment methods.[15] First-line treatment of MPEs includes chemotherapy aimed at tumor shrinkage and pleural fluid absorption. However, most NSCLC are or become chemoresistant. It has been found that an elevated level of VEGF contributes to angiogenesis and serous cavity effusions in cancer patients,[16] and formation of MPEs is associated with increased expression of vascular endothelial growth factor receptor by human lung cancer cells.[17] Since Avastin as a monoclonal antibody against VEGF has been developed for the treatment of NSCLC, whereas targeting the protein significantly inhibits the tumor growth,[18,19] it is reasonable to consider that efficacy of Avastin in treatment of MPEs with the increased survival rate was presumably related to its property, an angiogenesis inhibitor directed against VEGF production.[20–22] This result could provide important clues to Avastin's role in such MPE management and thus may offer a new therapy optimization to facilitate absorption of pleural fluid.
Concentration–response curve of paclitaxel in the pleural fluid were observed over time courses within 72 hours in presence and absence of Avastin. In contrast to the curve response to the paclitaxel treatment, the curve for the combination therapy sharply went down at the initial time of 0.5 until 24 hours in the graph, suggesting that Avastin had the potential to accelerate diffusion of the anticancer drug in arriving to the targeting tissues. In PK analysis, the values for Cmax were similar with the variability of <10% between the 2 treatment approaches, indicating that a peak concentration of paclitaxel in the pleural fluid was not influenced for the drug reaching to a specified area in the thoracic cavity.[23] The observation provided a basis of the statistic for further PK measures in the patients being studied.
Biological half-life of a substance can be complex due to factors including accumulation in tissues and active metabolites.[24] A value of T1/2α of the anticancer drug paclitaxel in the pleural fluid was determined based on the consideration that volume of distribution of a medication is an important parameter for assessing how effective a given drug regimen is, particularly when a new approach is being tested. The result manifested a 5.5-fold decrease in the value of T1/2α in the paclitaxel/Avastin-treated patients as compared to the paclitaxel-treated ones, indicating that intervention of Avastin was beneficial for facilitating diffusion of paclitaxel in achieving the targeting tissues. Avastin inhibits growth of blood vessels and reduces vessel permeability within the tumor tissues by binding to VEGF.[25] We assume that the rapid distribution of paclitaxel referred to the change in vessel permeability, since the decreased permeability would be beneficial for the anticancer drug paclitaxel remaining within the target tissues. In terms of T1/2β value in the same samples, our result displayed a 3-fold increase in the value in the paclitaxel/Avastin-treated patients as compared to the paclitaxel-treated ones, suggesting that the concentration of the chemotherapeutic drug paclitaxel may remain in a therapeutic level for a longer time. Since the elimination half-life refers to the speed at which the drug is eliminated through various bodily processes,[26] it is likely that the increase in extent of the half-life would promote the interaction between the anticancer drug and the tumor tissues. In support of the results on T1/2α and T1/2β, MRT of paclitaxel was determined by numerical data derived from the collected sample due to the fact that MRT of a drug often refers to the amount of time that a drug spends in the body.[27] Our result displayed a 2.4-fold increase in MRT in the paclitaxel/Avastin-treated patients, suggesting that the combination therapy was superior to the single treatment of paclitaxel in extending the duration of the action of the anticancer drug in the targeting tissues. The observation on MRT was completely consistent with the results of T1/2α and T1/2β, supporting the promise that the combination therapy not only accelerated early movement of the chemotherapeutic drug paclitaxel within the pleural fluid but also prolonged the therapeutic time of the drug in the targeting tissues.
In association with the PK study, serum VEGF levels and a 24-hour urinary excretion rate for the patients were examined. The results showed that the VEGF level significantly reduced in the Avastin-treated patients, not only demonstrating the activity of Avastin and its therapeutic effect but also displaying the benefit of the low VEGF level in improving efficacy of paclitaxel. Since metabolism of this anticancer drug remained unchanged in presence and absence of Avastin, it supported the consideration that an actual content of paclitaxel left its site of action was similar in the duration of the 2 treatments applied for the patients. In other words, Avastin enhanced the therapeutic effect of the anticancer drug paclitaxel on the patients due to prolonging the time of the drug molecules remaining in the target tissues. However, the finding only served as information corresponding to systemic clearance rate of the chemotherapy drug in treatment of MPEs, since the renal excretion for the drug paclitaxel is less than 14% of the total administered dose.[28]
Adverse events of drug responses were determined by according to the CTCAE v3.0.[29] Clinical manifestations and laboratory findings were compared between the patients who received the 2 approaches. Our results manifested that most of the treated patients had side effects ranked within the grades 1 to 2 except 1 in the paclitaxel/Avastin-treated patients having a low platelet count in the grade 3. The adverse events of low white blood cells and nausea/vomiting constituted a large-scale proportion in the study cohort with incidence of 50% and 38% in the paclitaxel-treated population and 50% and 50% in the patients who received the combination therapy. In contrast, there were no statistical differences in these clinical manifestations and laboratory findings between the presence and the absence of Avastin. These results led us to conclude that intervention with Avastin was feasible and safe in the process of treatment of MPEs. Paclitaxel has a significant clinical activity against a broad range of tumor types including lung cancer.[30] The antineoplastic agent interferes with the growth of both cancer cells and normal body cells, which are eventually destroyed with occurrence of some unwanted effects.[31] Since these side effects observed were common and not serious for the patients taking paclitaxel, it is conceivable that intervention of Avastin not only intensified the treatment effect of the anticancer drug in the patients but also shortened hospitalization time with reduced hospital costs.
In this study, we investigated efficacy of paclitaxel, in combination with Avastin, in treatment of MPEs, showing that intervention of Avastin was superior to paclitaxel used alone in improving treatment efficacy and survival rates. However, further studies regarding the effect of the combination therapy are needed to be acknowledged in a large cohort of the patients with MPEs.
Taken together, this study indicates that a combination therapy of paclitaxel and Avastin enhances efficacy in treatment of MPEs and a survival rate of the patients through inhibiting VERF production and prolonging the duration of the action of the anticancer drug in the targeting tissues.
Footnotes
Abbreviations: CR = complete relief, CT = computer technology, CTCAE = common terminology criteria for adverse events, IPC = indwelling pleural catheter, MPE = malignant pleural effusion, MRT = mean residence time, NSCLC = non–small cell lung cancer, OE = overall efficacy, PK = pharmacokinetic, VEGF = vascular endothelial growth factor.
NQ and FL have contributed equally to the article.
Funding/support: The work was supported by Beijing Municipal Commission of Science and Technology (Z131107002213040).
The authors have no conflicts of interest to disclose.
References
- [1].Neragi-Miandoab S. Malignant pleural effusion, current and evolving approaches for its diagnosis and management. Lung Cancer 2006;54:1–9. [DOI] [PubMed] [Google Scholar]
- [2].Antony VB, Loddenkemper R, Astoul P, et al. Management of malignant pleural effusions. Eur Respir J 2001;18:402–19. [DOI] [PubMed] [Google Scholar]
- [3].American Thoracic Society. Management of malignant pleural effusions. Am J Respir Crit Care Med 2000;162:1987–2001. [DOI] [PubMed] [Google Scholar]
- [4].Thomas JM, Musani AI. Malignant pleural effusions: a review. Clin Chest Med 2013;34:459–71. [DOI] [PubMed] [Google Scholar]
- [5].Roberts ME, Neville E, Berrisford RG, et al. Management of a malignant pleural effusion: British Thoracic Society Pleural Disease Guideline 2010. Thorax 2010;65suppl 2:ii32–40. [DOI] [PubMed] [Google Scholar]
- [6].Musani AI. Treatment options for malignant pleural effusion. Curr Opin Pulm Med 2009;15:380–7. [DOI] [PubMed] [Google Scholar]
- [7].Ferrara N, Hillan KJ, Novotny W. Bevacizumab (Avastin), a humanized anti-VEGF monoclonal antibody for cancer therapy. Biochem Biophys Res Commun 2005;333:328–35. [DOI] [PubMed] [Google Scholar]
- [8].Mukherji SK. Bevacizumab (Avastin). Am J Neuroradiol 2010;31:235–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Cheng D, Lee YC, Rogers JT, et al. Vascular endothelial growth factor level correlates with transforming growth factor-beta isoform levels in pleural effusions. Chest 2000;118:1747–53. [DOI] [PubMed] [Google Scholar]
- [10].Thickett DR, Armstrong L, Millar AB. Vascular endothelial growth factor (VEGF) in inflammatory and malignant pleural effusions. Thorax 1999;54:707–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Raben D, Helfrich B. Angiogenesis inhibitors: a rational strategy for radiosensitization in the treatment of non-small-cell lung cancer. Clin Lung Cancer 2004;6:48–57. [DOI] [PubMed] [Google Scholar]
- [12].Goldstraw P, Crowley JJ. The International Association for the Study of Lung Cancer international staging project on lung cancer. J Thorac Oncol 2006;1:281–6. [Google Scholar]
- [13].Schag CC, Heinrich RL, Ganz PA. Karnofsky performance status revisited: reliability, validity, and guidelines. J Clin Oncol 1984;2:187–93. [DOI] [PubMed] [Google Scholar]
- [14].Sugiura S, Ando Y, Minami H, et al. Prognostic value of pleural effusion in patients with non-small cell lung cancer. Clin Cancer Res 1997;3:47–50. [PubMed] [Google Scholar]
- [15].Chernow B, Sahn SA. Carcinomatous involvement of the pleura: an analysis of 96 patients. Am J Med 1977;63:695–702. [DOI] [PubMed] [Google Scholar]
- [16].Jia L, Du Y, Li T, et al. Differential expression of vascular endothelial growth factor-A, -C and -D for the diagnosis and prognosis of cancer patients with malignant effusions. Oncol Lett 2015;10:667–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yano S, Shinohara H, Herbst RS, et al. Production of experimental malignant pleural effusions is dependent on invasion of the pleura and expression of vascular endothelial growth factor/vascular permeability factor by human lung cancer cells. Am J Pathol 2000;157:1893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Das M, Wakelee H. Targeting VEGF in lung cancer. Expert Opin Ther Targets 2012;16:395–406. [DOI] [PubMed] [Google Scholar]
- [19].Pallis AG, Syrigos KN. Targeting tumor neovasculature in non-small-cell lung cancer. Crit Rev Oncol Hematol 2013;86:130–42. [DOI] [PubMed] [Google Scholar]
- [20].Hsu IL, Su WC, Yan JJ, et al. Angiogenetic biomarkers in non-small cell lung cancer with malignant pleural effusion: correlations with patient survival and pleural effusion control. Lung Cancer 2009;65:371–6. [DOI] [PubMed] [Google Scholar]
- [21].Das M, Wakelee H. Targeting VEGF in lung cancer. Expert Opin Ther Targets 2012;16:395–406. [DOI] [PubMed] [Google Scholar]
- [22].Horn L, Sandler AB. Emerging data with antiangiogenic therapies in early and advanced non-small-cell lung cancer. Clin Lung Cancer 2009;10:S7–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Urso R, Blardi P, Giorgi G. A short introduction to pharmacokinetics. Eur Rev Med Pharmacol Sci 2002;6:33–44. [PubMed] [Google Scholar]
- [24].Papanikolaou NC, Hatzidaki EG, Belivanis S, et al. Lead toxicity update. A brief review. Med Sci Monit 2005;11:RA329–36. [PubMed] [Google Scholar]
- [25].Shih T, Lindley C. Bevacizumab: an angiogenesis inhibitor for the treatment of solid malignancies. Clin Ther 2006;28:1779–802. [DOI] [PubMed] [Google Scholar]
- [26].Haley NJ, Sepkovic DW, Hoffmann D. Elimination of cotinine from body fluids: disposition in smokers and nonsmokers. Am J Public Health 1989;79:1046–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Li J, Tang J, Li Y, et al. Pharmacokinetic profile of paclitaxel in the plasma, lung, and diaphragm following intravenous or intrapleural administration in rats. Thorac Cancer 2015;6:45–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Sonnichsen DS, Relling MV. Clinical pharmacokinetics of paclitaxel. Clin Pharmacokinet 1994;24:256–69. [DOI] [PubMed] [Google Scholar]
- [29].Trotti A, Colevasb AD, Setser A, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 2003;13:176–91. [DOI] [PubMed] [Google Scholar]
- [30].Monsarrat B, Chatelut E, Royer I, et al. Modification of paclitaxel metabolism in a cancer patient by induction of cytochrome P450 3A4. Drug Metab Dispos 1998;26:229–33. [PubMed] [Google Scholar]
- [31].Sheppard BC, Rutten MJ, Meichsner CL, et al. Effects of paclitaxel on the growth of normal, polyposis, and cancerous human colonic epithelial cells. Cancer 1999;85:1451–64. [PubMed] [Google Scholar]


