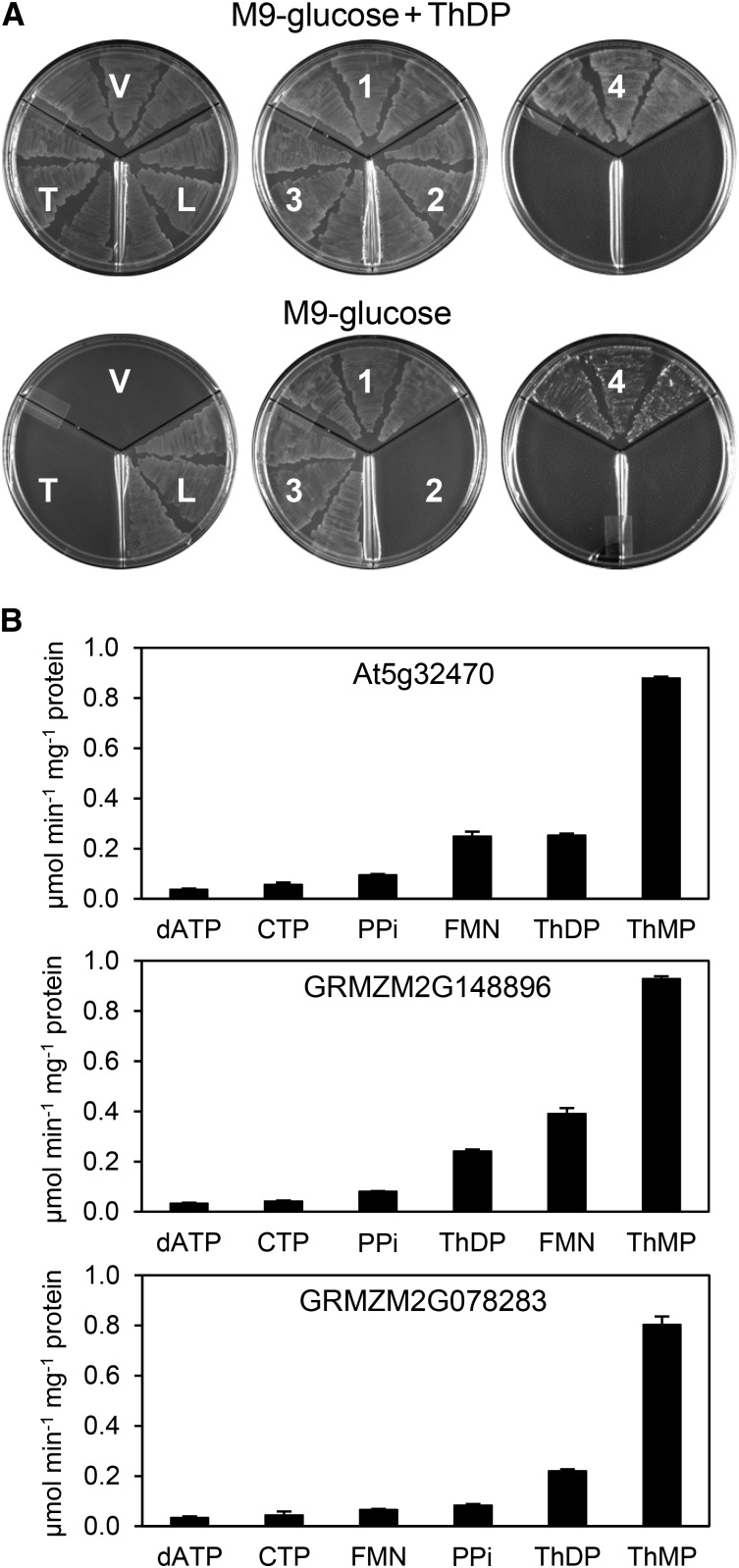
Figure 4.
Phosphatase Activities of the At5g32470 TenA-HAD Protein and Its Maize Orthologs.
(A) Evidence that the TenA-HAD proteins have in vivo ThMP phosphatase activity in E. coli and that the activity resides in the HAD domain. Three independent isolates of an E. coli ΔthiL strain transformed with vector alone (V) or harboring mouse TPK only (T), or mouse TPK plus native At5g32470 (1), the At5g32470 D317A mutant (2), maize GRMZM2G148896 (3), or maize GRMZM078283 (4) were plated on M9-glucose medium with or without 3.5 µM ThDP. The ΔthiL strain transformed with E. coli thiL (L) was included as a positive control. Plates contained 50 µg/mL chloramphenicol and 1 µg/mL anhydrotetracycline inducer. Images were captured after incubation for 24 h at 37°C. The predicted targeting sequences were removed from all the TenA-HAD proteins.
(B) Substrate profiles of recombinant TenA-HAD proteins, truncated to remove predicted targeting sequences. The panel of 95 substrates screened, and their abbreviations, are listed in Supplemental Table 1. Assays contained 0.25 mM substrate and 10 mM Mg2+. Data are means and se for four independent replicate assays. Activities against substrates not shown were <4% of that against ThMP for each enzyme.