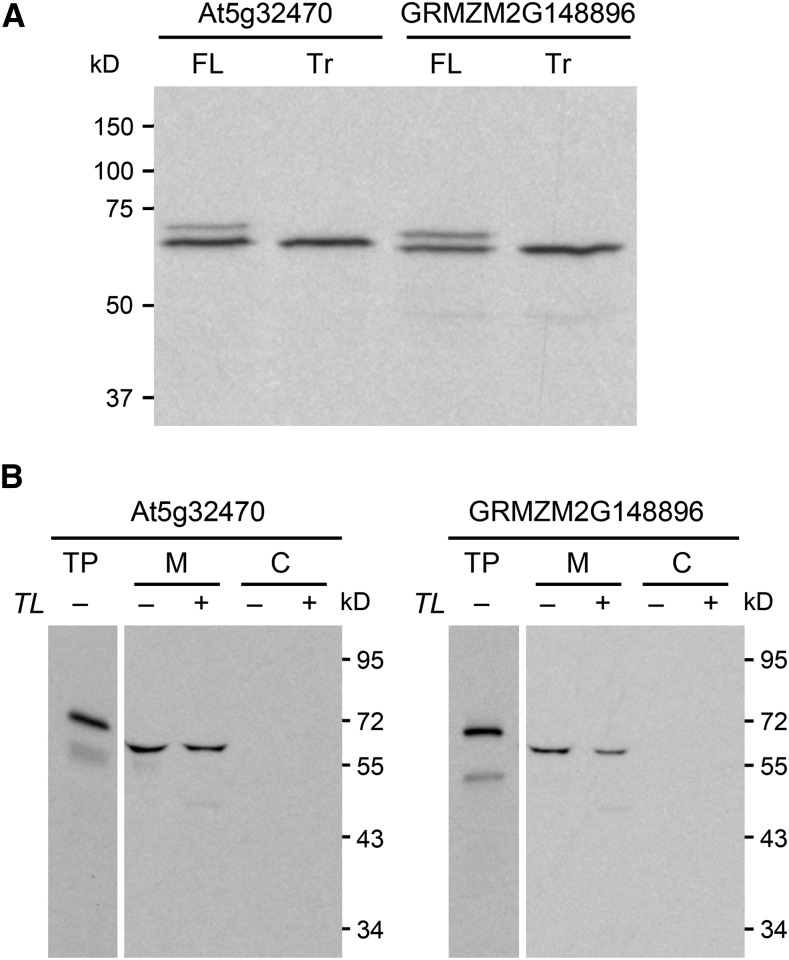
Figure 6.
In Vitro Evidence for Dual Translational Start Sites and Potential Mitochondrial Targeting of Arabidopsis and Maize TenA-HAD Proteins.
(A) In vitro-coupled transcription-translation identifies the first and second methionine codons as alternative start sites. Full-length (FL) At5g32470 and GRMZM2G148896 cDNAs and corresponding truncated (Tr) cDNAs starting at the second methionine codon (codon 47 and codon 44, respectively; Supplemental Figure 6) were transcribed and translated (in a wheat germ system) in the presence of [3H]leucine. Translation products were resolved by SDS-PAGE; radioactive bands were visualized by fluorography. Positions of molecular mass markers are indicated. All sequences were preceded by a strong Kozak translation initiation sequence (ACCATG…).
(B) The full-length At5g32470 and GRMZM2G148896 proteins are imported into and processed by mitochondria, but not chloroplasts. At5g32470 and GRMZM2G148896 full-length cDNAs preceded by native Kozak sequences were transcribed in vitro and translated (in a rabbit reticulocyte system) in the presence of [3H]leucine. The translation products (TP) were incubated in the light with mixed pea mitochondria (M) and chloroplasts (C). The organelles were mock treated (−) or thermolysin (TL) treated (+) to remove adsorbed proteins and then reisolated on a Percoll gradient. Proteins were separated by SDS-PAGE and visualized by fluorography. Samples were loaded on the basis of equal chlorophyll or mitochondrial protein content next to an aliquot of the translation product. Exposure times were adjusted to give comparable band intensities in all tracks. Positions of molecular mass markers (in kD) are shown. Note that the reticulocyte translation system, unlike the wheat germ system (Figure 6A), privileges the first translation start site, does not recognize the second, and apparently recognizes one or more minor, artifactual start sites within the sequence of the mature protein.