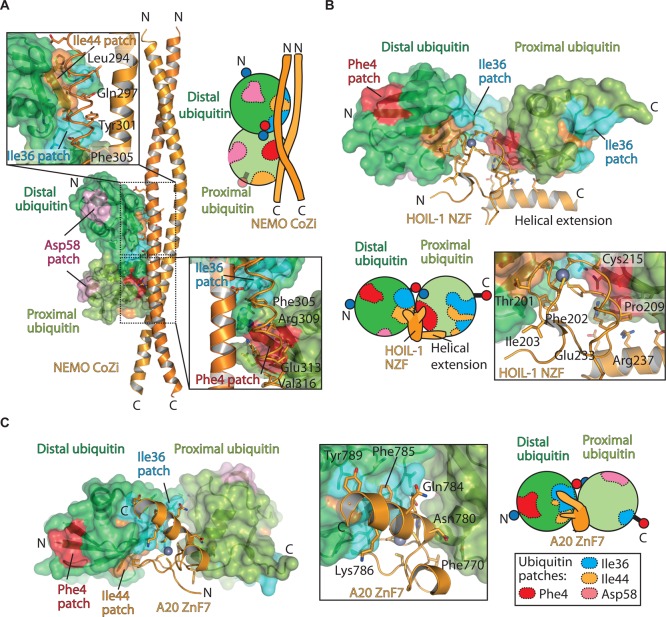
Figure 5. Molecular basis of Met1 recognition (‘readers’).
(A) Structure of mouse NEMO CoZi domain (orange) bound to Met1 diubiquitin (green surface) (PDB ID: 2ZVN) with different ubiquitin patches highlighted as in Figure 1B. Insert, close-up view of both distal and proximal ubiquitin interactions. For clarity, one CoZi domain is shown as a cartoon, while the other domain is shown as a ribbon to highlight the side chains that interact with the ubiquitin moieties. (B) Structure of the HOIL-1 NZF bound to Met1-linked diubiquitin (PDB ID: 3B0A). The HOIL-1 NZF domain is shown as a cartoon (orange) with Met1 diubiquitin as a surface with different patches coloured. Insert, the Zn co-ordinating loop that engages with both distal and proximal ubiquitin moieties is shown. (C) Structure of A20 ZnF7 bound to Met1-linked diubiquitin (PDB ID: 3VUW). Insert, residues from A20 ZnF7 that interact with the distal and proximal ubiquitin moieties are shown.