Abstract
Type III secretion systems (T3SS) are central virulence factors for many pathogenic Gram-negative bacteria, and secreted T3SS effectors can block key aspects of host cell signaling. To counter this, innate immune responses can also sense some T3SS components to initiate anti-bacterial mechanisms. The Yersinia pestis T3SS is particularly effective and sophisticated in manipulating the production of pro-inflammatory cytokines IL-1β and IL-18, which are typically processed into their mature forms by active caspase-1 following inflammasome formation. Some effectors, like Y. pestis YopM, may block inflammasome activation. Here we show that YopM prevents Y. pestis induced activation of the Pyrin inflammasome induced by the RhoA-inhibiting effector YopE, which is a GTPase activating protein. YopM blocks YopE-induced Pyrin-mediated caspase-1 dependent IL-1β/IL-18 production and cell death. We also detected YopM in a complex with Pyrin and kinases RSK1 and PKN1, putative negative regulators of Pyrin. In contrast to wild-type mice, Pyrin deficient mice were also highly susceptible to an attenuated Y. pestis strain lacking YopM, emphasizing the importance of inhibition of Pyrin in vivo. A complex interplay between the Y. pestis T3SS and IL-1β/IL-18 production is evident, involving at least four inflammasome pathways. The secreted effector YopJ triggers caspase-8- dependent IL-1β activation, even when YopM is present. Additionally, the presence of the T3SS needle/translocon activates NLRP3 and NLRC4-dependent IL-1β generation, which is blocked by YopK, but not by YopM. Taken together, the data suggest YopM specificity for obstructing the Pyrin pathway, as the effector does not appear to block Y. pestis-induced NLRP3, NLRC4 or caspase-8 dependent caspase-1 processing. Thus, we identify Y. pestis YopM as a microbial inhibitor of the Pyrin inflammasome. The fact that so many of the Y. pestis T3SS components are participating in regulation of IL-1β/IL-18 release suggests that these effects are essential for maximal control of innate immunity during plague.
Author Summary
Many pathogenic Gram-negative bacteria express type III secretion systems (T3SS) that translocate bacterial proteins into host cells with the potential of altering normal cell processes. Yersinia pestis, the causative agent of plague, harbors a T3SS which is particularly effective in suppressing innate immunity and release of pro-inflammatory cytokines IL-1β and IL-18, potent triggers of anti-bacterial responses. These cytokines are produced via processing by active caspase-1 in inflammasome complexes. Pyrin is an inflammasome component that recognizes alterations in certain host cell signals. Here we show that the T3SS effector protein YopM inhibits effector YopE-mediated Pyrin-induced caspase-1 activation, IL-1β, IL-18 and cell death triggered by Y. pestis. We also found that blocking the Pyrin pathway is important for disease development in a mouse model of bubonic plague. Thus, YopM is a microbial molecule blocking Pyrin inflammasomes.
Introduction
Type III secretion systems (T3SS) are essential virulence factors of many pathogenic Gram-negative bacteria. These systems include a needle-like structure, translocon proteins that form a pore with which the needle can dock in the membrane of host target cells, and a set of secreted effector proteins delivered to the target cell cytoplasm through the docked needle. The effector proteins exert control over key cellular processes that contribute to antibacterial defenses or pathogenesis, including immune signaling, phagocytosis, and induction of cell death. In response, the innate immune system has evolved the ability to recognize a number of T3SS components and initiate protective inflammatory responses when they are detected. In some T3SS-dependent pathogens that cause severe disease—like Y. pestis, the causative agent of plague—the balance between these opposing activities strongly favors the bacteria.
As we and others have shown, a key strategy of Y. pestis is preventing production of active IL-1β and IL-18 through an apparent combination of activities [1,2,3,4,5,6]. Maturation of these major pro-inflammatory cytokines is primarily dependent on processing by the protease caspase-1. In turn, activation of pro-caspase-1 depends on assembly of multiprotein intracellular complexes known as inflammasomes, triggered by recognition of the bacterial products or activities via NLR proteins or other alternative pathways. Although the fully intact T3SS of Y. pestis with its seven secreted Yersinia outer protein (Yop) effectors (YopM, E, K, J, T, H and YpkA) blocks caspase-1 activity effectively, some components of this system are themselves inflammasome activators if the system is incomplete [2,3,7,8], able to trigger anti-bacterial effects [2,5,9]. Thus, to be effective in regulating inflammation, the T3SS must suppress the effects of the same pro-inflammatory signaling systems that it activates. We believe that this small effector toolkit, heavily dedicated towards immune evasion and conferring high virulence [10], makes Yersinia an excellent model for characterizing T3SS functions as well as host immune pathways.
In the absence of all seven secreted effector proteins, Y. pestis producing the T3SS needle and pore-forming translocon pore proteins (YopB, D) activates the NLRP3/ NLRC4 inflammasome pathways effectively, possibly by hypertranslocation of T3SS pore and rod components [3,11]. This activation is blocked by addition of the effector YopK, which can regulate influx of Yops [3,11]. The effector YopJ triggers a non-canonical RIP1-caspase-8-caspase-1 inflammasome pathway [7,12], and also can inhibit NF-κB, MAP2K and MAP3K, reducing synthesis of pro-IL-1β/IL-18. The activation of caspase-8 by YopJ also triggers apoptosis. Loss of YopJ in combination with loss of a second effector, YopM, results in high levels of active caspase-1 and IL-1β/IL-18, comparable to that seen with a strain lacking all seven effectors [1]. YopM was originally proposed to be a caspase-1 inhibitor [4], although an alternative model for YopM inhibition of caspase-1, involving other proteins, has recently been proposed [13]. The precise action of YopM on caspase-1 activation is thus unclear.
Here we report that Y. pestis YopM is unable to inhibit T3SS-triggered caspase-1 activation mediated by NLRP3, NLRC4, or caspase-8. Instead, this effector inhibits another signal occurring through a Pyrin-dependent pathway. Pyrin (also called MEFV, TRIM20 or marenostrin) is the founding member of the pyrin domain family of proteins. A number of mutations in human Pyrin have been reported and associated with the most common human autoinflammatory disease, Familial Mediterranean Fever (FMF), where the pathology is believed to be initiated by hyperactivation of Pyrin-Asc-caspase-1 inflammasomes [14,15,16]. Bacteria can also activate Pyrin inflammasomes. It was recently proposed that covalent modifications of RhoA GTPase by bacterial toxins and type 6 secretion systems (T6SS), resulting in RhoA inhibition, triggering activation of Pyrin-mediated production of mature IL-1β/IL-18 [17]. YopM is the first specific microbial inhibitor of this incompletely understood pathway to be reported. We also present evidence that the Y. pestis effector YopE, a Rho inhibitor and GTPase activating protein (GAP), triggers Pyrin inflammasomes. We suggest that inhibition of this pathway by YopM is a central feature of inflammasome suppression observed during Y. pestis infection.
Results
YopM inhibits the Pyrin inflammasome, which is activated by YopE
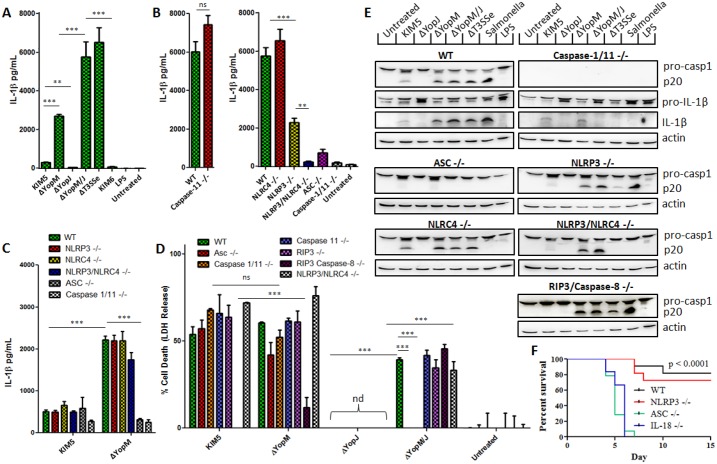
Many of the Yersinia T3SS effectors have inhibitory effects on immune functions. Y. pestis YopM is considered a suppressor of innate immunity, although mechanisms by which it acts are not clear. Both we and others have shown that YopM is an inhibitor of caspase-1 activation [1,4,6,13]. A YopM deletion in Y. pestis KIM5, normally expressing a fully functional T3SS encoded on the pCD1 plasmid, triggers increased levels of active caspase-1 and IL-1β in mouse primary bone-marrow derived macrophages (BMDM), indicating that YopM suppresses a bacteria-triggered inflammasome pathway, and an additional deletion of YopJ further increases IL-1β release (Fig 1A, [1]). The KIM6 strain, which does not harbor pCD1 and thus lacks the entire T3SS, triggered minimal IL-1β release. In contrast, we observed a strong IL-1β signal in response to the ΔT3SSe strain, lacking secreted effectors but expressing the basic T3SS machinery such as rod/needle/translocon components (Fig 1A). Both NLRP3 and NLRC4 partially contributed to sensing the presence of T3SS needle/translocon (Fig 1B), in addition to caspase-1 and the adaptor Asc. To identify the pathway inhibited by Y. pestis YopM, we tested whether BMDMs lacking specific inflammasome components would fail to increase IL-1β production when YopM is absent (Fig 1C). Although YopM inhibits a pathway dependent on Asc and caspase-1 (Fig 1C, [1]), we observed no decrease of IL-1β in cells lacking NLRP3, NLRC4, or caspase-11 compared to wild-type cells after infection with Y. pestis lacking YopM. We also tested cells lacking NLRP12, RIP3, or caspase-8 (S1 Fig), and found none of these proteins to be required for the IL-1β producing pathway which YopM suppresses. Many factors influence bacterial triggering of inflammasomes via T3SS, the stimulation conditions utilized in this project differ from an earlier publication [2] and may not robustly favor activation of the NLRP12 pathway. At present we do not have evidence for how the NLRP12 pathway is triggered and how it interacts with the T3SS-mediated pathways discussed in this manuscript. The same pattern was observed for cell death (Fig 1D); YopM inhibits caspase-1 dependent cell death (pyroptosis) [1], but this cell death still occurs in the absence of the inflammasome components tested above (Fig 1C).
Fig 1. The Y. pestis effector YopM suppresses a different IL-1β-producing pathway than the one triggered by the needle/translocon through NLRP3 and NLRC4.
IL-1β in supernatants from A) WT LPS-primed BMDMs infected with Y. pestis Yop mutant strains, B) BMDMs of indicated genotypes infected with ΔT3SSe, or C) LPS-primed BMDMs infected with KIM5 and ΔYopM were measured by ELISA 6 hrs p.i. (MOI 10). D) Cell death was assayed by LDH release in LPS-primed BMDMs infected with indicated strains at 6 hrs p.i. (MOI 10). Figures are representative of three or more experiments. E) Total protein from LPS-primed BMDMs infected with indicated strains (combined cell lysate and supernatant) was separated by SDS-PAGE and analyzed by Western Blot for IL-1β and caspase-1. F) Mice of indicated genotypes were injected s.c. with 160 CFU of KIM1001ΔM/J and monitored for survival past 21 days. P value for survival comparisons reflect differences between WT (n = 11) or NLRP3 KO (n = 11) and IL-18 (n = 6), Asc KO (n = 14). Shown is mean plus s.d. for triplicate wells. ND: not detected. A-E are representative of three experiments or more, F representative of two experiments performed. * p<0.05, **p<0.01, ***p<0.001.
Furthermore, we performed experiments directly comparing effects on caspase-1 cleavage (Fig 1E). YopJ suppresses pro-IL-1β and pro-caspase-1 production [1,6], and triggers a relatively small amount of IL-1β processing in a caspase-8-dependent manner [7]. We noted that caspase-1 may not be absolutely required for caspase-8 dependent IL-1β processing in response to parental Y. pestis KIM5 (Fig 1E), arguing that caspase-8 can act independently to cleave IL-1β following YopJ action. Caspase-1 activation by Y. pestis expressing the needle/translocon, but lacking all secreted effectors (ΔT3SSe) is fully dependent on NLRP3/NLRC4, while YopM inhibits caspase-1 processing and IL-1β release independently of NLRP3, NLRC4, NLRP12, RIP3, or caspase-8 (Fig 1E, S1 Fig). It is however a noteworthy point that in NLRP3/NLRC4 deficient cells, Y. pestis ΔYopM/J triggers a substantial amount of caspase-1 and IL-1β processing while ΔT3SSe (which additionally lacks the other 5 translocated Yops) does not. Taken together, these results indicate that YopM inhibits an Asc-dependent inflammasome triggered by another Yop effector. We have reported that deletion of both YopM and YopJ in a fully virulent Y. pestis KIM1001 strain implicates increased IL-1β and IL-18 in vivo, and leads to significant attenuation following subcutaneous (s.c.) infection mimicking bubonic plague [1]. Here we report that this attenuation is dependent upon Asc but not NLRP3 (Fig 1F), consistent with our in vitro data.
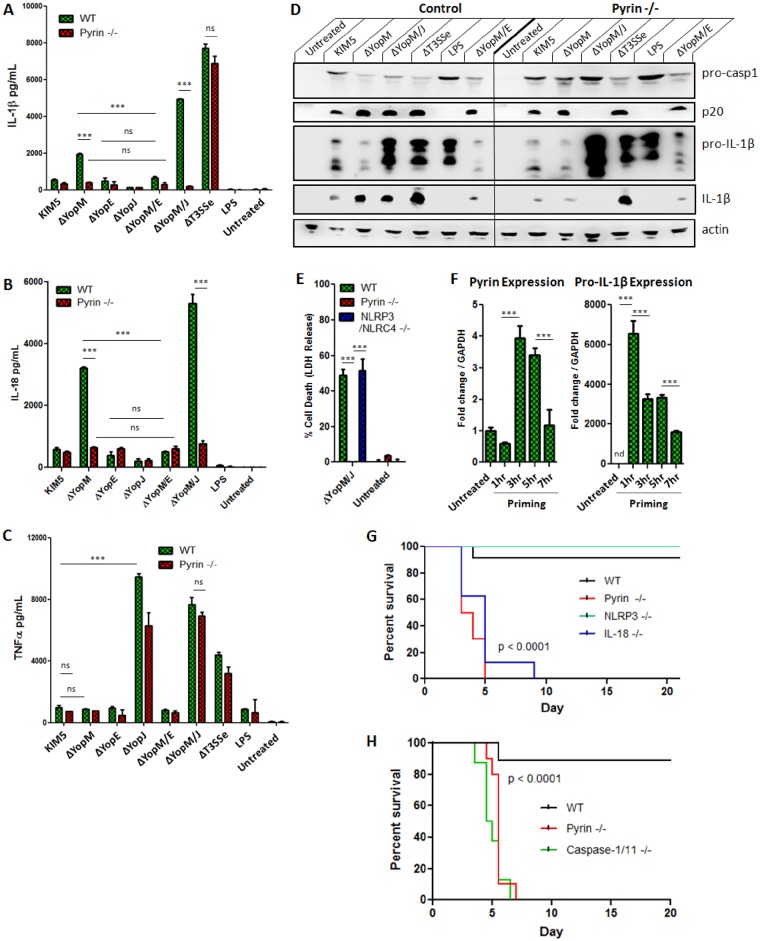
We previously suggested YopE as a potential activator of the YopM-inhibited pathway, as removing YopE from the KIM5 ΔYopM strain abolished all the IL-1β blocked by YopM [1]. One key feature of YopE is that this effector inhibits Rho family GTPases via its inherent GAP activity [18,19]. It should be noted that some macrophage anti-bacterial responses induced by YopE have been suggested to be dependent upon its GAP mimetic ability [20]. One remaining candidate for a participant in an Asc-dependent YopM-inhibited inflammasome is Pyrin [21], which has been linked to anti-bacterial innate immunity following RhoA GTPase covalent modification and inhibition [17]. When we tested wild-type BMDMs and BMDMs lacking Pyrin, we found that the IL-1β and IL-18 induction inhibited by YopM is fully dependent on Pyrin, and appears to be triggered by YopE (Fig 2A and 2B). This is comparable to the pathway driving IL-1β induced by Clostridium difficile toxin TcdB (S2 Fig). By contrast, TNFα secretion was not appreciably impacted (Fig 2C). Caspase-1 activation (Fig 2D) and pyroptosis (Fig 2E) associated with ΔYopM, in particular when the caspase-8 activating effector YopJ was additionally deleted, were strongly reduced in the absence of Pyrin. Thus, we propose a model where YopE, by its GAP activity inhibits Rho GTPases, and triggers Pyrin activation that is blocked by YopM. Future experiments will determine which Rho GTPases are involved in YopE-triggered caspase-1 cleavage via Pyrin. Priming appears necessary for Y. pestis ΔYopM to induce increased levels of IL-1β compared to the parental strain [1,4,6,13] (S3 Fig). This may be partly explained by the increased expression of Pyrin in the presence of TLR stimulation or killed Y. pestis (Fig 2F, S3 Fig), as baseline levels of Pyrin in macrophages may be low [22,23], although upregulation of Pyrin is delayed compared to IL-1β (Fig 2F). However, we cannot exclude the possibility that the regulation of other pathway members also plays a role. We also found that macrophages lacking the transcription factor C/EBPβ [24] were unable to produce IL-1β specifically in response to KIM5ΔYopM or KIM5ΔYopM/J (S4 Fig), and we note that transcription of Pyrin is controlled by C/EBPβ [25]. To test the hypothesis that the inhibition of the Pyrin pathway in vivo contributes to virulence, we infected WT, Pyrin KO, NLRP3 KO and IL-18 KO mice with the attenuated Y. pestis KIM1001 ΔYopM/J strain. For the fully virulent Y. pestis KIM1001 strain, the deletion of both these Yops is necessary for attenuation and increased IL-1b/IL-18 production in vivo [1]. We found that the attenuation of this Y. pestis strain lacking YopM, apparent in wild-type C57Bl/6 mice, could be completely reversed in Pyrin KO mice. These animals were highly susceptible to infection and all succumbed within a few days (Fig 2G), similar to caspase-1/11 deficient mice (Fig 2H). YopM is a strong inhibitor of Pyrin-mediated inflammasome activation, and we propose that the inhibition of this innate immunity pathway is a key feature of bacterially driven anti-host responses during plague, thus emphasizing the in vivo importance of our in vitro findings.
Fig 2.
LPS-primed BMDMs were infected with indicated Y. pestis strains for 6 hours; A) IL-1β, B) IL-18, and C) TNFα were measured in supernatants by ELISA 6 hrs p.i. (MOI 10); D) Total protein from LPS-primed BMDMs infected with indicated strains (combined cell lysate and supernatant) was separated by SDS-PAGE and analyzed by Western Blot for IL-1β and caspase-1. E) Cell death was assayed by LDH release in LPS-primed BMDMs infected with indicated strains at 6 hrs p.i. (MOI 10). F) Expression of Pyrin and Pro-IL-1β mRNA was measured by RT-PCR at 1, 3, 5, or 7 hours after addition of 100ng/mL LPS to WT BMDMs. G) WT C57Bl/6 (n = 12), Pyrin KO (n = 10), NLRP3 KO (n = 7) or IL-18 KO (n = 8) or H) WT (n = 9), Pyrin KO (n = 10) or caspase-1/11 KO (n = 8) mice were infected s.c. with Y. pestis KIM1001 ΔYopM/J (150 CFU) and monitored for survival up to 21 days. G, H): P value reflects comparison of WT vs Pyrin KO, NLRP3 vs Pyrin KO, WT vs IL-18 KO or WT vs caspase-1/11 KO. Figures are representative of three or more experiments, G, F are representative of two experiments. Shown is mean plus s.d. for triplicate wells. * p<0.05, **p<0.01, ***p<0.001.
YopK, but not YopM, keeps NLRP3 and NLRC4 activation by the Y. pestis needle/translocon in check
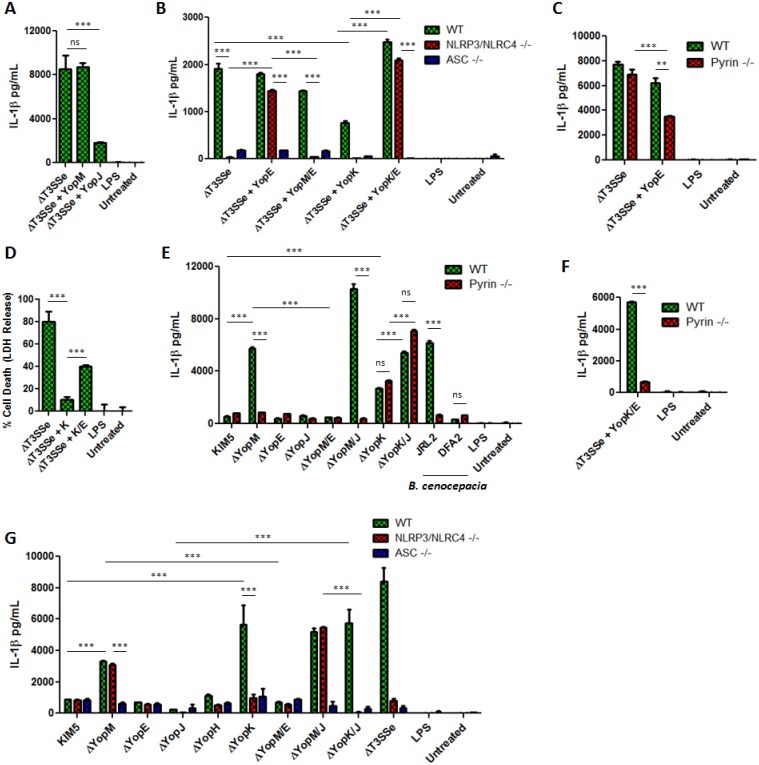
As we were unsure whether effector YopK would inhibit Pyrin, we conducted a set of experiments probing involvement of YopK in the Y. pestis-triggered NLRP3/NLRC4 pathways compared to the Pyrin pathway. We confirmed the dependence of Y. pestis needle/translocon-mediated IL-1β secretion on caspase-1, NLRP3 and NLRC4 (Fig 1), suggested by studies in Y. pseudotuberculosis [3,11]. We observed a strong IL-1β induction in response to the ΔT3SSe lacking secreted effectors (Fig 1B). As reported previously, TNFα production is not significantly affected by the presence of the needle/translocon as compared to a strain (KIM6) lacking all T3SS components [1]. We next investigated whether reconstituting endogenous levels of YopM, YopK or YopJ on a ΔT3SSe background (inserted back onto the T3SS containing pCD1 plasmid by allelic exchange) would inhibit IL-1β release. The ΔT3SSe + YopJ strain demonstrated strong but incomplete inhibition of IL-1β, suggesting that inhibition of transcription may be a dominant response to YopJ in this case, and not the YopJ inflammasome activating ability. Importantly, ΔT3SSe + YopM had similar IL-1β induction as the ΔT3SSe (Fig 3A). This is consistent with the hypothesis that YopM inhibits the Pyrin inflammasome (Fig 2) but may not in this condition be a general inhibitor of caspase-1 [4]. We furthermore observed that Pyrin deficient macrophages released similar amounts of IL-1β in response to the needle/translocon expressing ΔT3SSe strain, emphasizing that the response to the presence of basic T3SS nano-machinery components requires NLRP3/NLRC4 but is independent of Pyrin. YopE may be an activator of the Pyrin pathway (Fig 2), and indeed, when expressing YopE in the ΔT3SSe strain the IL-1β release became less dependent upon NLRP3/NLRC4 (Fig 3B), and partly Pyrin dependent (Fig 3C). The NLRP3/NLRC4 dependence was fully restored when YopM was expressed in addition to YopE (Fig 3B). This suggests that YopM inhibits a YopE-triggered Pyrin pathway which is distinct from the needle/translocon triggered NLRP3/NLRC4 pathway.
Fig 3.
A-F,E,G) IL-1β was measured by ELISA in supernatants of LPS-primed BMDMs infected for 6 hours with indicated bacterial strains, or D) cell death was measured by LDH release at 6 hours p.i. (MOI 10). Figures are representative of three or more experiments. Shown is mean plus s.d. for triplicate wells. * p<0.05, **p<0.01, ***p<0.001.
Earlier data suggested that YopK may limit NLRP3 activation, either by preventing hypertranslocation of the pore-forming complex or regulating the injection of Yops [3,11]. Indeed, the addition of YopK reduces ΔT3SSe-triggered IL-1β effectively (Fig 3B), and also prevents ΔT3SSe induced cell death(Fig 3D). Interestingly, upon expression of the proposed Pyrin activator YopE in ΔT3SSe in addition to YopK, the IL-1β release became essentially independent of NLRP3/NLRC4 (Fig 3B) but fully dependent upon Pyrin (Fig 3E and 3F). When YopK is deleted from a KIM5 or KIM5 ΔYopJ background, we observed a sharp rise in IL-1β production and cell death compared to parental Y. pestis KIM5 both in primary macrophages and dendritic cells (Fig 3E, S5 Fig). This IL-1β production in response to infection with KIM5 ΔYopK was eliminated in NLRP3/NLRC4 deficient cells, but was independent of Pyrin (Fig 3F and 3G). We conclude that YopK is both necessary and sufficient to block inflammasome activation induced by the presence of the Y. pestis needle/translocon via NLRP3/NLRC4, but does not appear to block Pyrin activation induced by YopE. We cannot fully exclude the possibility that impact on translocation of other molecules by YopK and YopE [11,26,27,28,29] could be a factor in our observations. In contrast, IL-1β induced by the ΔYopM strain is fully dependent upon the presence of Pyrin, similarly to a Burkholderia cenocepacia strain (JRL2) that lacks a functional T3SS but expresses a Pyrin-activating T6SS (Fig 3E) [15,30,31,32]. For B. cenocepacia, the absence of the T6SS in the DFA2 strain strongly reduces the ability to trigger IL-1β release, in spite of a functional T3SS, underscoring how Yersinia and Burkholderia trigger Pyrin inflammasomes via different secretion systems. The picture that emerges is consistent with the hypothesis that YopM is an inhibitor of the Pyrin inflammasome triggered by YopE, whereas YopK inhibits NLRP3/NLRC4 activation triggered by the presence of the T3SS needle/translocon. This emphasizes a new complexity in the host inflammasome activation triggered by the Y. pestis T3SS.
Pyrin is in a complex with YopM and RSK1, PKN1 kinases
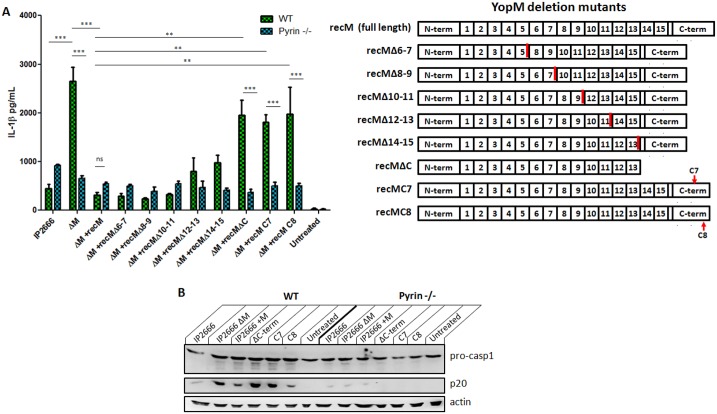
Y. pestis is a close relative to Y. pseudotuberculosis, a human enteric pathogen. We confirmed that Y. pseudotuberculosis also induced IL-1β via Pyrin in the absence of YopM (Fig 4A). YopM has been proposed to interact with kinases of the PKN and RSK families. YopM consists of multiple leucine-rich repeat (LRR) domains, and several deletions of these LRR domains have been generated [33]. Using a Y pseudotuberculosis IP2666 strain reconstituted (rec) with YopM mutants on a ΔYopM background [33], we determined that the C-terminus of YopM is needed to inhibit Pyrin-mediated IL-1β release and caspase-1 processing (Fig 4A and 4B). In fact, alanine substitutions of only the last three C-terminal YopM amino acids in the recM C8 strain essentially prevented the ability of YopM to block IL-1β production (Fig 4A). This is also the region of YopM necessary for interaction with RSK1 kinase, [34], and these domains partly overlap with YopM regions interacting with PKN1 [34].
Fig 4.
A) IL-1β was measured by ELISA in supernatants of LPS-primed BMDMs infected for 6 hours with Y. pseudotuberculosis IP2666, including strains expressing YopM with partial deletions (MOI 10). The numbers in the YopM protein refer to different leucine-rich repeat (LRR) domains of YopM, and C-term indicates the C-terminal end. RecM indicates reconstitution (rec) of IP2666 ΔYopM with variants of YopM, as shown in the figure. C7 and C8 are two different triple alanine substitutions near the C-terminus of YopM [33]. B) Total protein from LPS-primed BMDMs infected with indicated strains (combined cell lysate and supernatant) was separated by SDS-PAGE and analyzed by Western Blot for IL-1β and caspase-1. Figures are representative of two independent experiments. Shown is mean plus s.d. for triplicate wells. * p<0.05, **p<0.01, ***p<0.001.
As human and mouse pyrin exhibit some differences in structure, we wanted to confirm whether YopM is capable of inhibiting IL-1β induction in human cells. To do this, we isolated peripheral blood mononuclear cells (PBMCs) and infected them with Y. pestis. For this experiment, cells were not primed as PBMCs are known to produce IL-1β in response to TLR stimuli alone in an ERK-dependent manner [35]. We confirmed that YopM also contributes to inhibition of IL-1β secretion in human PBMCs (Fig 5A).
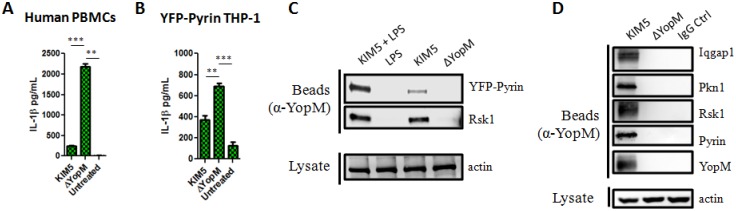
Fig 5. YopM maintains an inhibitory phenotype in human PBMCs, and in a human THP-1 cell line overexpressing YFP-Pyrin.
Co-IP pulldown in these cells as well as mouse BMDMs indicate YopM interaction with Pyrin, Rsk1, Pkn1, and Iqgap1. A) PBMCs were isolated from healthy human donor blood and infected at MOI 10 with indicated Y. pestis strains without priming. At 6 hours p.i. supernatant was collected for IL-1β detection by ELISA. B) Cultured YFP-Pyrin THP-1 cells were differentiated with 100nM Vitamin D3 for 48–72 hours, and infected with indicated Y. pestis strains at MOI 10. Shown is IL-1β assayed from supernatants by ELISA at 6 hrs p.i. Figures are representative of three or more experiments. Shown is mean plus s.d. for triplicate wells. * p<0.05, **p<0.01, ***p<0.001. C-D) Shown are Western blot results of co-IP with anti-YopM using C) Vitamin D3-differentiated, unprimed YFP-Pyrin cells or D) LPS-primed BMDMs after infection with the indicated strains at MOI 10 for 3 hours. Bead-bound protein and lysates were separated by SDS-PAGE and analyzed by Western Blot for the proteins indicated.
We next tested the effect of YopM in a monocytic human THP-1 cell line expressing YFP-Pyrin, this line was chosen as matured THP-1 cells have minimal endogenous levels of Pyrin. We found an IL-1β secretion pattern generally comparable to human PBMCs, where YopM is also capable of inhibiting IL-1β production (Fig 5B). We used the THP-1 YFP-Pyrin cells as a tool for biochemical analysis of how YopM or Rsk1 interacts with Pyrin [33,34,36]. Rsk1, and recently the cytoskeletal scaffolding protein Iqgap1, have been suggested to be important for caspase-1 inhibition by YopM [13]. Pull-down assays in THP-1 YFP-Pyrin cells and mouse BMDMs using anti-YopM antibody showed that YopM interacts with a complex containing Rsk1, PKN1, Pyrin and Iqgap1 (Fig 5C and 5D). As we also detected Pyrin in the bound fraction, it may directly or indirectly (via the kinases) interact with YopM.
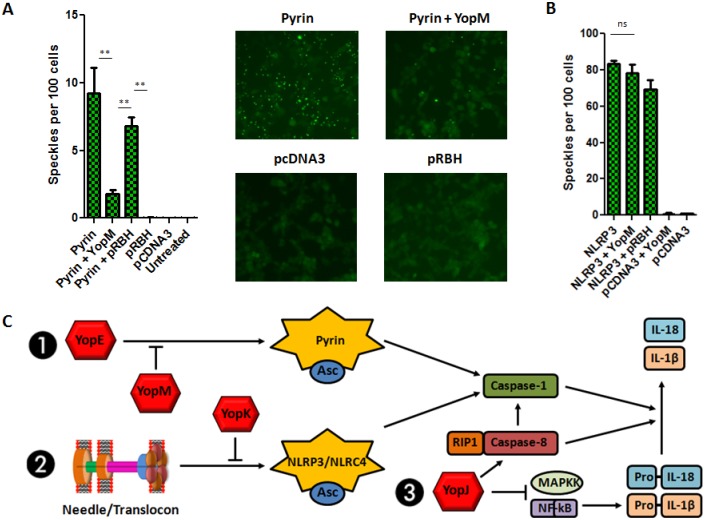
Next, we tested the ability of YopM alone to inhibit Pyrin activation. HEK293T cells stably expressing Asc-YFP were transiently transfected with plasmids encoding Pyrin, YopM, or both constructs together. We observed significantly increased Asc complex (speckle) formation upon transfection of Pyrin, visualized as numerous fluorescent puncta, indicating inflammasome assembly following Pyrin over-expression (Fig 6A). This Asc speckling was significantly reduced upon co-transfection of YopM. The expression of NLRP3 also triggered the formation of Asc-speckles, but was not affected by the co-expression of YopM (Fig 6B), suggesting YopM specificity for inhibiting the Pyrin pathway.
Fig 6. YopM prevents the formation of Pyrin-dependent but not NLRP3-dependent Asc complexes.
A) HEK293T cells stably expressing Asc-YFP were transfected with pCDNA3-Pyrin, pRBH-YopM, or both constructs together. B) pCDNA3-NLRP3 and respective empty vectors were used as positive and negative controls. Asc speckles were visualized, quantified, and normalized to cell number. Figures are representative of three or more experiments. Shown is mean plus s.d. for triplicate fields quantified. * p<0.05, **p<0.01, ***p<0.001. C) Proposed model integrating the major interactions of the Y. pestis T3SS with inflammasome pathways.
Discussion
Taken together, our data show complex interactions of a bacterial T3SS with host inflammasome signaling pathways. We have identified several pathways triggered by Y. pestis T3SS, as both Pyrin, caspase-8 and NLRP3/NLRC4 appear directly and distinctly involved in regulation of caspase-1 cleavage and IL-1β release. Two secreted T3SS effectors, YopK and YopM, appear to be specific inhibitors of the NLRP3/NLRC4 activation induced by the presence of the T3SS needle/translocon, and the YopE-induced Pyrin activation, respectively. Inflammasome activation by YopE may represent a process where the host innate immune system acquired the ability to sense damaging microbial interference and inhibition of a specific pathway (RhoA signaling). Our current model of how the Y. pestis T3SS components intersect with specific inflammasome pathways is shown in Fig 6C. Both YopK and YopM are participating in maximal suppression of innate immunity following Y. pestis infection, and are central components of the arsenal that this highly virulent pathogen allocates to interference with key anti-bacterial immune responses. Our findings highlight the remarkable sophistication that Yersinia displays when interfacing with inflammasome signals.
Previously, covalent modifications of RhoA by bacteria were proposed to trigger Pyrin activation [17]. Our data suggest an additional way that bacteria initiate Pyrin-dependent mechanisms, as we here propose that the GAP effector YopE activate the cascade leading to Pyrin activation. Thus, changes in the Rho GTPase phosphorylation status could trigger the cascade leading to Pyrin-mediated IL-1β release and cell death. It would be interesting to see whether other bacterial GAPs also harbor this ability, such as Salmonella enterica serovar Typhimurium effector SptP [37,38], although this effector has not been suggested to target RhoA. We also cannot exclude the possibility that other Rho family modifying effectors, such as Y. pestis YopT which cleaves Rho GTPases, also can contribute, although in Y. pestis, the deletion of YopE fully abolishes the additional IL-1β triggered by the ΔYopM strain. We can also extend the discussion of possible Pyrin pathway modifiers to consider bacterial Rho GTPase exchange factors (GEFs), activating Rho family proteins, as players in the system [37]. Indeed, the symmetry axis between YopE and YopM that simultaneously activates and inhibits signaling mediated by RhoGTPases also has parallels in other pathogens. Of note, S. Typhimurium expresses both SptP (with GAP activity towards Rac1) and SopE-SopE2 (with GEF activity), highlighting the complex regulation of cellular activation by bacterial T3SS effectors. For both the YopE/YopM and SptP/SopE-SopE2 sets, the inhibiting effectors may be necessary only when the activating effector is present. This type of observation may also open up for hypotheses about sequential evolution of specific effector proteins. The requirement for the inhibitor YopM only in the presence of the activator YopE suggests that the latter may have been acquired first, and hence hints at the evolutionary steps that produced the extant system. More generally, it reveals interplay between the host and pathogen that may in part drive the selection for increasing complexity of Type III secretion systems: addition of a new effector is favored because it counters the host responses driven by the one most recently acquired.
Other aspects that can impact Pyrin signaling include mutations in Pyrin itself [16,39], mutations in mouse WDR proteins impacting actin depolymerization [40], and alterations of the mevalonate pathway [41,42] which can activate Pyrin inflammasomes. This implies that a number of different ways to trigger Pyrin may exist, but this also widens the range of potential microbial or pathological impact of this pathway. Also, historical plague pandemics in Europe started in the same area where FMF is most prevalent, in the Mediterranean basin. Although difficult to verify, it is possible that some FMF-related mutations caused altered susceptibility to infection, and this could have contributed to modified host responses to bacteria [43].
Several regulators of the Pyrin inflammasome pathway have recently been proposed, including 14-3-3 proteins [39] and PKN1/2 kinases [41]. Interestingly, PKN kinases also bind to YopM, opening up for future studies of direct roles of these interactions on Pyrin activation. Here we also show that RSK1 kinase binds a complex of YopM, PKN1 and Pyrin. One attractive model involves phosphorylation of Pyrin [39,41] by YopM-interacting kinases and stabilization of the inactivated and phosphorylated Pyrin by 14-3-3 proteins. The complex may also involve the scaffold protein Iqgap1 [13]. This model may also include one or more phosphatases that will de-phosphorylate Pyrin under stimulating conditions, although such a phosphatase has not yet been identified.
Very recently, a paper was published showing that YopM from Y. pseudotuberculosis inhibits the Pyrin inflammasome triggered by YopE [44]. Both that study and ours demonstrate that attenuated YopM or YopM/J mutant strains re-gain virulence in vivo in the absence of Pyrin. Similar to the mechanism what we suggest with Y. pestis, the report indicated that Y. pseudotuberculosis YopM interacts with kinases PKN1/2 and RSK1 in a complex with Pyrin, but also that the kinases phosphorylate Pyrin which in turn is stabilized in a phosphorylated inactive state by 14-3-3 proteins [44]. One difference between the two papers is that Chung et al also suggests that YopT, via its protease activity towards RhoA, also can trigger Pyrin activation. Although we have not studied YopT in detail, it appears from our experiments that on a Y. pestis deltaYopM background, YopE fully accounts for the Pyrin activating ability and that a dual deletion of YopM and YopE brings IL-1β release and caspase-1 cleavage down to the level of the parental strain. Some of these differences may be explained by differences in YopE GAP activity between Y. pestis and Y. pseudotuberculosis, or by differences in experimental conditions.
Another possibility is higher basal inflammasome activation mediated by Y. pestis KIM YopJ via caspase-8, which may also explain why the parental Y. pestis strain has a fairly strong IL-1β release and caspase-1 cleavage (Figs 1 and 2) compared to the low (Fig 4) or absent [44] caspase-1 cleavage or IL-1β release observed with Y. psedotuberculosis. This difference may very well be explained by the markedly higher enzymatic activity of Y. pestis KIM YopJ compared to Y. pseudotuberculosis YopJ, as previously proposed [8]. Nevertheless, despite small differences mentioned above in between these two reports, we can summarize that Y. psedotuberculosis and Y. pestis YopM proteins both engage in similar inhibitory activity towards Pyrin-dependent inflammasome activation, and are central in the strategy of both pathogens to inhibit innate immune responses in their favor.
In conclusion, we have identified Y. pestis YopM as a microbial inhibitor of the Pyrin inflammasome pathway. Detailed knowledge about the mechanisms that the T3SS effector YopM influences may open up for the development of novel treatments in Pyrin-mediated diseases.
Methods
Mice
Most mouse strains used in this study were described previously [2,24]. Pyrin (Mefv) -/- mice lacking exons 1–4 mice were generated at Genentech from gene- targeted C57BL/6N C2 ES cells. Alternatively, Pyrin -/- mice provided by Jackson Laboratories were utilized. BMDMs were differentiated from bone marrow harvested from the femurs of 6–20 week old mice. Mice were injected s.c. with 160 CFU KIM1001ΔYopM/J and monitored for survival.
Bacterial strains and growth conditions
The fully virulent KIM1001 strain of Y. pestis, the pgm-deficient but pCD1+ strain KIM5 (containg the full T3SS) as well as its mutant derivatives (ΔYopJ, ΔYopM, ΔYopM/J, ΔT3SSe and the KIM6 strain entirely lacking the T3SS-encoding plasmid pCD1), and the Y. pseudotuberculosis IP2666ΔYopM as well as IP2666ΔYopM+recM (reconstituted with YopM variants) mutant strains were previously described [1,5,7,45,46]. The strains were generated by in-frame deletions and allelic exchange. The T3SS secreted effector deficient strain (ΔT3SSe) was constructed by making sequential in-frame deletions on KIM5 [1]. This strain lacks Yops M, E, J, H, T, K and YpkA, but expresses pore-forming translocon components Yops B, D and the machinery necessary to assemble a T3SS needle/rod. The full-length genes of yopK, yopM, or yopE were restored onto the ΔT3SSe background on the pCD1 plasmid. Y. pestis strains were grown in tryptose-beef extract broth (2xYT for Y. pseudotuberculosis strains) with 2.5mM CaCl2. Bacteria were added to cells at MOI 10.
Cell stimulations
Bone marrow derived macrophages (BMDMs) from mice in our facility were differentiated in RPMI 1640 supplemented with 10% fetal calf serum (FCS), 25mM HEPES, 10ug/mL ciprofloxacin, and 10% L929 conditioned medium containing M-CSF for 5 days [1]. Cells were primed with 100 ng/mL E. coli O111:B4 LPS for 5 hours or allowed to rest in antibiotic-free RPMI with 10% FCS and 25mM HEPES without antibiotic before addition of bacteria at an MOI of 10. IL-1β/IL-18 Elisa, LDH cell death assays and immunoprecipitation and western blots were performed as described in supplemental material. HEK293 Asc-YFP cells were provided by K. Fitzgerald and THP-1-Pyrin-YFP cells from M. Wewers and M. Gavrilin [32]. Human PBMC were obtained from donor whole blood (harvested in our lab at UMass) using Lymphoprep density gradient (Axis-Shield) and stimulated in RPMI 1640 supplemented with 10% FCS and 25mM HEPES. TcdA and TcdB were from List Biological Labs.
Statistical analysis
In vitro assays were analyzed by two-way ANOVA followed by Bonferroni post-test. Differences in mouse survival were analyzed by Kaplan-Meyer analysis and logrank test. Values where p < 0.05 were considered significant.
Ethics statement
All animal studies were performed in compliance with the federal regulations set forth in the Animal Welfare Act (AWA), the recommendations in the Guide for the Care and Use of Laboratory Animals of the National Institutes of Health, and the guidelines of the UMass Medical School Institutional Animal Use and Care Committee. All protocols used in this study were approved by the Institutional Animal Care and Use Committee at the UMass Medical School (protocols A-2332 and A-2339). Human PBMC were obtained from healthy volunteer donor whole blood collected in our lab at UMass Medical School after donor review of information fact sheet and oral consent. The research enrolled only adult subjects, and all provided informed consent. In accordance with U.S. Code of Federal Regulations 45 CFR 46.117(c)(2), the UMass Medical School Institutional Review Board approved oral consent and waived written documentation of consent as the research and phlebotomy presented no more than minimal risk of harm to subjects and involved no procedures for which written consent is normally required outside of the research context. The consent of each individual was recorded as demographic information according to requirements by the National Institutes of Health. All human subject work was conducted in accordance with the guidelines given by the Institutional Review Board at UMass Medical School, and approved by the same Board (protocol H-11183).
More detailed methods are found in S1 Supporting information.
Supporting Information
(PDF)
(DOCX)
(DOCX)
LPS-primed BMDMs were infected with indicated strains of Y. pestis at MOI 10 for 6 hours, and supernatant IL-1β was assayed by ELISA in A) WT, RIP3/Caspase-8 -/- and B) WT, NLRP12 -/-, RIP3 -/-, BMDMs. Decrease of IL-1 release in the absence of caspase-8 cannot be explained by reduced caspase-1 cleavage (Fig 1), but rather reflects reduced transcriptional activity. C) Total protein from LPS-primed RIP3 -/- BMDMs infected with indicated strains (combined cell lysate and supernatant) was separated by SDS-PAGE and analyzed by Western Blot for caspase-1.
(TIF)
LPS-primed (100 ng/ml) BMDMs of indicated genotypes (WT C57Bl/6 or KO) were treated with 0.2uM TcdA, 0.2uM TcdB, or 5mM ATP. A) supernatant IL-1β was assayed by ELISA and B) cell death was assayed by LDH assay.
(TIF)
Priming can be achieved with LPS or heat-killed bacteria expressing either hexa- or tetra-acylated LPS. The suppressive action of YopJ appears to contribute to the need for priming. A) 100ng/mL LPS or 1x108 CFU equivalents of heat-killed KIM5 were added to BMDMs either 5 hours before infection, or simultaneously with live KIM5 or ΔYopM at MOI 10. Supernatant from 6 hours p.i. was assayed for IL-1β by ELISA. B) Priming can be achieved with heat-killed Y. pestis regardless of whether it is grown at 26°C or 37°C, despite expression of tetra-acylated LPS with low stimulatory ability. C) Unprimed BMDMs were infected with indicated strains of Y. pestis (temperature-shifted) at MOI 10 for 6 hours, and supernatant IL-1β was assayed by ELISA. It is also worth noting that without priming, KIM5ΔYopM produces IL-1β comparable to parental KIM5, whereas KIM5ΔYopM/J triggers significantly elevated levels of IL-1β (S3 Fig). It is possible that YopJ suppresses priming that occurs during the course of the 6-hour infection, either by inhibiting NF-κB- or MAPK mediated gene expression, or by inducing apoptosis before sufficient priming can occur. This is further suggested by the fact that LPS-priming is not required to elicit a strong IL-1β response with KIM5ΔYopM/J, unlike KIM5ΔYopM where YopJ is present.
(TIF)
LPS-primed BMDMs were infected with indicated strains of Y. pestis at MOI 10 for 6 hours, and supernatant IL-1β was assayed by ELISA.
(TIF)
LPS-primed BMDCs were infected with indicated strains of Y. pestis at MOI 10 for 6 hours; A) supernatant IL-1β was assayed by ELISA, and B) cell death was measured by LDH release.
(TIF)
Acknowledgments
We thank Gail Germain, Karen Saylor and Nancy Martin for animal husbandry and genotyping.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The work was supported by National Institutes of Health Grants AI07538 and U19 AI057319 (to EL), AI095213 (to DR), HL076278 (to MAG and MDW), AR055398 (to ESA), AI113166 (to JM), AI110695 (to DW), the Norwegian Cancer Society, and Research Council of Norway Center of Excellence Funding Scheme Project 223255/F50 (to EL). The research of PFJ was supported in part by the Intramural Research Program of the NIH, National Cancer Institute, Center for Cancer Research. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the U.S. Government. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. URLs: https://www.nih.gov/, https://kreftforeningen.no/, http://www.forskningsradet.no/en/Home_page/1177315753906, http://www.cancer.gov/
References
- 1. Ratner D, Orning MP, Starheim KK, Marty-Roix R, Proulx MK, et al. (2016) Manipulation of IL-1beta and IL-18 production by Yersinia pestis effectors YopJ and YopM and redundant impact on virulence. J Biol Chem. 291: 9894–9905. 10.1074/jbc.M115.697698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Vladimer GI, Weng D, Paquette SW, Vanaja SK, Rathinam VA, et al. (2012) The NLRP12 inflammasome recognizes Yersinia pestis. Immunity 37: 96–107. 10.1016/j.immuni.2012.07.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Brodsky IE, Palm NW, Sadanand S, Ryndak MB, Sutterwala FS, et al. (2010) A Yersinia effector protein promotes virulence by preventing inflammasome recognition of the type III secretion system. Cell Host Microbe 7: 376–387. 10.1016/j.chom.2010.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. LaRock CN, Cookson BT (2012) The Yersinia virulence effector YopM binds caspase-1 to arrest inflammasome assembly and processing. Cell Host Microbe 12: 799–805. 10.1016/j.chom.2012.10.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Montminy SW, Khan N, McGrath S, Walkowicz MJ, Sharp F, et al. (2006) Virulence factors of Yersinia pestis are overcome by a strong lipopolysaccharide response. Nat Immunol 7: 1066–1073. 10.1038/ni1386 [DOI] [PubMed] [Google Scholar]
- 6. Schoberle TJ, Chung LK, McPhee JB, Bogin B, Bliska JB (2016) Uncovering an Important Role for YopJ in the Inhibition of Caspase-1 in Activated Macrophages and Promoting Yersinia pseudotuberculosis Virulence. Infect Immun. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Weng D, Marty-Roix R, Ganesan S, Proulx MK, Vladimer GI, et al. (2014) Caspase-8 and RIP kinases regulate bacteria-induced innate immune responses and cell death. Proc Natl Acad Sci U S A 111: 7391–7396. 10.1073/pnas.1403477111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Zheng Y, Lilo S, Brodsky IE, Zhang Y, Medzhitov R, et al. (2011) A Yersinia effector with enhanced inhibitory activity on the NF-kappaB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages. PLoS Pathog 7: e1002026 10.1371/journal.ppat.1002026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Vladimer GI, Marty-Roix R, Ghosh S, Weng D, Lien E (2013) Inflammasomes and host defenses against bacterial infections. Curr Opin Microbiol 16: 23–31. 10.1016/j.mib.2012.11.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Viboud GI, Bliska JB (2005) Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis. Annu Rev Microbiol 59: 69–89. 10.1146/annurev.micro.59.030804.121320 [DOI] [PubMed] [Google Scholar]
- 11. Zwack EE, Snyder AG, Wynosky-Dolfi MA, Ruthel G, Philip NH, et al. (2015) Inflammasome activation in response to the Yersinia type III secretion system requires hyperinjection of translocon proteins YopB and YopD. MBio 6: e02095–02014. 10.1128/mBio.02095-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Philip NH, Dillon CP, Snyder AG, Fitzgerald P, Wynosky-Dolfi MA, et al. (2014) Caspase-8 mediates caspase-1 processing and innate immune defense in response to bacterial blockade of NF-kappaB and MAPK signaling. Proc Natl Acad Sci U S A 111: 7385–7390. 10.1073/pnas.1403252111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chung LK, Philip NH, Schmidt VA, Koller A, Strowig T, et al. (2014) IQGAP1 is important for activation of caspase-1 in macrophages and is targeted by Yersinia pestis type III effector YopM. MBio 5: e01402–01414. 10.1128/mBio.01402-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Manukyan G, Aminov R (2016) Update on Pyrin Functions and Mechanisms of Familial Mediterranean Fever. Front Microbiol 7: 456 10.3389/fmicb.2016.00456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Yang J, Xu H, Shao F (2014) Immunological function of familial Mediterranean fever disease protein Pyrin. Sci China Life Sci 57: 1156–1161. 10.1007/s11427-014-4758-3 [DOI] [PubMed] [Google Scholar]
- 16. Chae JJ, Cho YH, Lee GS, Cheng J, Liu PP, et al. (2011) Gain-of-function Pyrin mutations induce NLRP3 protein-independent interleukin-1beta activation and severe autoinflammation in mice. Immunity 34: 755–768. 10.1016/j.immuni.2011.02.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Xu H, Yang J, Gao W, Li L, Li P, et al. (2014) Innate immune sensing of bacterial modifications of Rho GTPases by the Pyrin inflammasome. Nature 513: 237–241. 10.1038/nature13449 [DOI] [PubMed] [Google Scholar]
- 18. Vlahou G, Schmidt O, Wagner B, Uenlue H, Dersch P, et al. (2009) Yersinia outer protein YopE affects the actin cytoskeleton in Dictyostelium discoideum through targeting of multiple Rho family GTPases. BMC Microbiol 9: 138 10.1186/1471-2180-9-138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Black DS, Bliska JB (2000) The RhoGAP activity of the Yersinia pseudotuberculosis cytotoxin YopE is required for antiphagocytic function and virulence. Mol Microbiol 37: 515–527. [DOI] [PubMed] [Google Scholar]
- 20. Wang X, Parashar K, Sitaram A, Bliska JB (2014) The GAP activity of type III effector YopE triggers killing of Yersinia in macrophages. PLoS Pathog 10: e1004346 10.1371/journal.ppat.1004346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Yu JW, Wu J, Zhang Z, Datta P, Ibrahimi I, et al. (2006) Cryopyrin and pyrin activate caspase-1, but not NF-kappaB, via ASC oligomerization. Cell Death Differ 13: 236–249. 10.1038/sj.cdd.4401734 [DOI] [PubMed] [Google Scholar]
- 22. Gavrilin MA, Mitra S, Seshadri S, Nateri J, Berhe F, et al. (2009) Pyrin critical to macrophage IL-1beta response to Francisella challenge. J Immunol 182: 7982–7989. 10.4049/jimmunol.0803073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gavrilin MA, Wewers MD (2011) Francisella Recognition by Inflammasomes: Differences between Mice and Men. Front Microbiol 2: 11 10.3389/fmicb.2011.00011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Sterneck E, Tessarollo L, Johnson PF (1997) An essential role for C/EBPbeta in female reproduction. Genes Dev 11: 2153–2162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Papin S, Cazeneuve C, Duquesnoy P, Jeru I, Sahali D, et al. (2003) The tumor necrosis factor alpha-dependent activation of the human mediterranean fever (MEFV) promoter is mediated by a synergistic interaction between C/EBP beta and NF kappaB p65. J Biol Chem 278: 48839–48847. 10.1074/jbc.M305166200 [DOI] [PubMed] [Google Scholar]
- 26. Dewoody RS, Merritt PM, Marketon MM (2013) Regulation of the Yersinia type III secretion system: traffic control. Front Cell Infect Microbiol 3: 4 10.3389/fcimb.2013.00004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dewoody R, Merritt PM, Marketon MM (2013) YopK controls both rate and fidelity of Yop translocation. Mol Microbiol 87: 301–317. 10.1111/mmi.12099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Dewoody R, Merritt PM, Houppert AS, Marketon MM (2011) YopK regulates the Yersinia pestis type III secretion system from within host cells. Mol Microbiol 79: 1445–1461. 10.1111/j.1365-2958.2011.07534.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Sheahan KL, Isberg RR (2015) Identification of mammalian proteins that collaborate with type III secretion system function: involvement of a chemokine receptor in supporting translocon activity. MBio 6: e02023–02014. 10.1128/mBio.02023-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Aubert DF, Hu S, Valvano MA (2015) Quantification of type VI secretion system activity in macrophages infected with Burkholderia cenocepacia. Microbiology 161: 2161–2173. 10.1099/mic.0.000174 [DOI] [PubMed] [Google Scholar]
- 31. Rosales-Reyes R, Aubert DF, Tolman JS, Amer AO, Valvano MA (2012) Burkholderia cenocepacia type VI secretion system mediates escape of type II secreted proteins into the cytoplasm of infected macrophages. PLoS One 7: e41726 10.1371/journal.pone.0041726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gavrilin MA, Abdelaziz DH, Mostafa M, Abdulrahman BA, Grandhi J, et al. (2012) Activation of the pyrin inflammasome by intracellular Burkholderia cenocepacia. J Immunol 188: 3469–3477. 10.4049/jimmunol.1102272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. McCoy MW, Marre ML, Lesser CF, Mecsas J (2010) The C-terminal tail of Yersinia pseudotuberculosis YopM is critical for interacting with RSK1 and for virulence. Infect Immun 78: 2584–2598. 10.1128/IAI.00141-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. McPhee JB, Mena P, Bliska JB (2010) Delineation of regions of the Yersinia YopM protein required for interaction with the RSK1 and PRK2 host kinases and their requirement for interleukin-10 production and virulence. Infect Immun 78: 3529–3539. 10.1128/IAI.00269-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Ghonime MG, Shamaa OR, Das S, Eldomany RA, Fernandes-Alnemri T, et al. (2014) Inflammasome priming by lipopolysaccharide is dependent upon ERK signaling and proteasome function. J Immunol 192: 3881–3888. 10.4049/jimmunol.1301974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Hentschke M, Berneking L, Belmar Campos C, Buck F, Ruckdeschel K, et al. (2010) Yersinia virulence factor YopM induces sustained RSK activation by interfering with dephosphorylation. PLoS One 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Aktories K (2011) Bacterial protein toxins that modify host regulatory GTPases. Nat Rev Microbiol 9: 487–498. 10.1038/nrmicro2592 [DOI] [PubMed] [Google Scholar]
- 38. Fu Y, Galan JE (1999) A salmonella protein antagonizes Rac-1 and Cdc42 to mediate host-cell recovery after bacterial invasion. Nature 401: 293–297. 10.1038/45829 [DOI] [PubMed] [Google Scholar]
- 39. Masters SL, Lagou V, Jeru I, Baker PJ, Van Eyck L, et al. (2016) Familial autoinflammation with neutrophilic dermatosis reveals a regulatory mechanism of pyrin activation. Sci Transl Med 8: 332ra345. [DOI] [PubMed] [Google Scholar]
- 40. Kim ML, Chae JJ, Park YH, De Nardo D, Stirzaker RA, et al. (2015) Aberrant actin depolymerization triggers the pyrin inflammasome and autoinflammatory disease that is dependent on IL-18, not IL-1beta. J Exp Med 212: 927–938. 10.1084/jem.20142384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Park YH, Wood G, Kastner DL, Chae JJ (2016) Pyrin inflammasome activation and RhoA signaling in the autoinflammatory diseases FMF and HIDS. Nat Immunol 17: 914–921. 10.1038/ni.3457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Akula MK, Shi M, Jiang Z, Foster CE, Miao D, et al. (2016) Control of the innate immune response by the mevalonate pathway. Nat Immunol 17: 922–929. 10.1038/ni.3487 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Ross JJ (2007) Goats, germs, and fever: Are the pyrin mutations responsible for familial Mediterranean fever protective against Brucellosis? Med Hypotheses 68: 499–501. 10.1016/j.mehy.2006.07.027 [DOI] [PubMed] [Google Scholar]
- 44. Chung LK, Park YH, Zheng Y, Brodsky IE, Hearing P, et al. (2016) The Yersinia Virulence Factor YopM Hijacks Host Kinases to Inhibit Type III Effector-Triggered Activation of the Pyrin Inflammasome. Cell Host Microbe 20: 296–306. 10.1016/j.chom.2016.07.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sodeinde OA, Subrahmanyam YV, Stark K, Quan T, Bao Y, et al. (1992) A surface protease and the invasive character of plague. Science 258: 1004–1007. [DOI] [PubMed] [Google Scholar]
- 46. McCoy AJ, Koizumi Y, Toma C, Higa N, Dixit V, et al. (2010) Cytotoxins of the human pathogen Aeromonas hydrophila trigger, via the NLRP3 inflammasome, caspase-1 activation in macrophages. Eur J Immunol 40: 2797–2803. 10.1002/eji.201040490 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(DOCX)
(DOCX)
LPS-primed BMDMs were infected with indicated strains of Y. pestis at MOI 10 for 6 hours, and supernatant IL-1β was assayed by ELISA in A) WT, RIP3/Caspase-8 -/- and B) WT, NLRP12 -/-, RIP3 -/-, BMDMs. Decrease of IL-1 release in the absence of caspase-8 cannot be explained by reduced caspase-1 cleavage (Fig 1), but rather reflects reduced transcriptional activity. C) Total protein from LPS-primed RIP3 -/- BMDMs infected with indicated strains (combined cell lysate and supernatant) was separated by SDS-PAGE and analyzed by Western Blot for caspase-1.
(TIF)
LPS-primed (100 ng/ml) BMDMs of indicated genotypes (WT C57Bl/6 or KO) were treated with 0.2uM TcdA, 0.2uM TcdB, or 5mM ATP. A) supernatant IL-1β was assayed by ELISA and B) cell death was assayed by LDH assay.
(TIF)
Priming can be achieved with LPS or heat-killed bacteria expressing either hexa- or tetra-acylated LPS. The suppressive action of YopJ appears to contribute to the need for priming. A) 100ng/mL LPS or 1x108 CFU equivalents of heat-killed KIM5 were added to BMDMs either 5 hours before infection, or simultaneously with live KIM5 or ΔYopM at MOI 10. Supernatant from 6 hours p.i. was assayed for IL-1β by ELISA. B) Priming can be achieved with heat-killed Y. pestis regardless of whether it is grown at 26°C or 37°C, despite expression of tetra-acylated LPS with low stimulatory ability. C) Unprimed BMDMs were infected with indicated strains of Y. pestis (temperature-shifted) at MOI 10 for 6 hours, and supernatant IL-1β was assayed by ELISA. It is also worth noting that without priming, KIM5ΔYopM produces IL-1β comparable to parental KIM5, whereas KIM5ΔYopM/J triggers significantly elevated levels of IL-1β (S3 Fig). It is possible that YopJ suppresses priming that occurs during the course of the 6-hour infection, either by inhibiting NF-κB- or MAPK mediated gene expression, or by inducing apoptosis before sufficient priming can occur. This is further suggested by the fact that LPS-priming is not required to elicit a strong IL-1β response with KIM5ΔYopM/J, unlike KIM5ΔYopM where YopJ is present.
(TIF)
LPS-primed BMDMs were infected with indicated strains of Y. pestis at MOI 10 for 6 hours, and supernatant IL-1β was assayed by ELISA.
(TIF)
LPS-primed BMDCs were infected with indicated strains of Y. pestis at MOI 10 for 6 hours; A) supernatant IL-1β was assayed by ELISA, and B) cell death was measured by LDH release.
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.