Abstract
Over the last decade, cryoballoon ablation has emerged as an effective alternate strategy to point-by-point radiofrequency ablation for treatment of symptomatic atrial fibrillation. There are several reasons for this. First, the acute and long-term safety and efficacy associated with cryoablation appear comparable to that of radiofrequency ablation in patients with both paroxysmal and also persistent atrial fibrillation. Second, cryoablation offers certain advantages over conventional radiofrequency ablation including a gentler learning curve, shorter ablation and procedure times as well as lack of need for costly electroanatomical mapping technologies commonly utilized with radiofrequency ablation. Lastly, with the recent advent of the second-generation cryoballoon, the effectiveness of cryoablation has further improved dramatically. This comprehensive review examines the gradual evolution of the cryoablation tools as well as the rationale and data in support of the currently-available cryoballoon technologies for catheter ablation of atrial fibrillation.
Keywords: Atrial Fibrillation, Catheter Ablation, Cryoablation, Cryoballoon, Outcome
Introduction
Catheter ablation has emerged as a practical approach for treatment of symptomatic atrial fibrillation (AF) in those who fail membrane-stabilizing antiarrhythmic drug (AAD) therapy.[1] AF ablation has been shown to improve patient quality of life[2] and reduce hospital readmission.[3] Additionally, the observed benefits even persist in patients in whom complete freedom from AF cannot be achieved.[2] As the role for catheter ablation in the management of AF has evolved within the last 2 decades, so have the ablative techniques and strategies. To date, a variety of energy modalities have been utilized for catheter ablation of AF including unipolar radiofrequency (RF),[4] irrigated[5] and non-irrigated bipolar RF,[6] laser,[7] cryothermy,[8] and high-intensity focused ultrasound.[9] While the long-term safety and efficacy of RF energy has rendered it the mainstay of arrhythmia ablation therapy, there are certain practical and theoretical advantages to using cryoenergy.
The principles of cryobiology were first established with investigations on the treatment of frostbite and tumor destruction.[10] Current data suggests that a temperature of −30°C to −40°C is necessary to induce cell death.[10] Ice formation is the cornerstone of cellular injury with cryotherapy which occurs both intracellularly and extracellularly. Hypothermy-induced cellular and tissue destruction occurs through immediate and delayed mechanisms.[10] The immediate effects including hypothermic injury and cellular freeze rupture are mediated through tissue freezing/thawing, whereas delayed injury results from vasculature damage and apoptosis or programmed cell death.[11]
Rationale For Using Cryoenergy For AF Ablation
As a result of technological advances, evolutions in catheter design, and improved energy delivery that have come about in the last two decades, cryoablation is now routinely used in cardiac electrophysiology laboratories. Cryotherapy exploits the Joule–Thomson effect[12] to achieve temperatures between −30°C to −90°C at the catheter–tissue interface.[13] Furthermore, the use of cryoablation for pulmonary (PV) vein isolation may offer certain advantages. First, tissue-catheter adhesion during cryoablation can result in improved catheter stability. Second, cryoablation is associated with reduced pain and discomfort since the afferent pain fibers are ‘frozen’ as opposed to stimulated thermally.[14] Third, cryoablation carries a lower risk of thrombus formation and consequently systemic thromboembolization and stroke, since it is associated with decreased activation of platelets and the coagulation cascade as compared with RF.[15] Fourth, cryoablation leaves the connective tissue matrix intact and also avoids the risk of steam pops.[14] Fifth, the lack of circulation, vascular disruption, and endothelial injury at the center of the cryolesion results in uniform tissue necrosis.[14] As a result, unlike with RF, cryolesions consist of a smooth, sharply-demarcated necrotic core corresponding to the frozen volume within the zone of lethality, and they are thought to be associated with reduced likelihood of ulceration, stenosis, and formation of fistulas and strictures.[14] Nonetheless, cryoablation can still pose a significant threat to collateral structures such as the esophagus, lungs, coronary arteries, and phrenic and vagus nerves.[13] Another disadvantage relates to the impact of blood flow on lesion size. That is, increased blood flow surrounding the ablation catheter can significantly attenuate the size of cryolesions. As such, cryoablation is generally more effective in ‘low-flow’ areas.[12] Meanwhile, thaw time continues to remain the most important determinant of acute and long-term efficacy associated with cryoablation.[16,17]
Focal Cryoablation Of AF
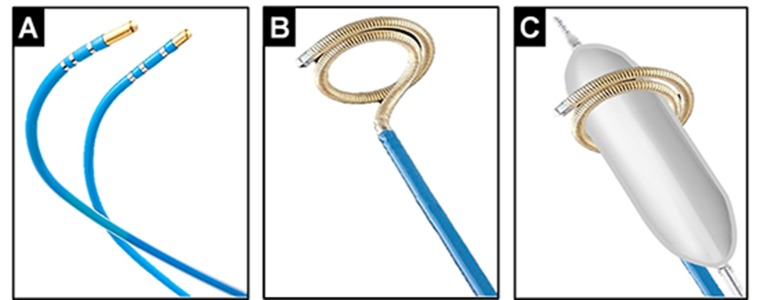
The safety and feasibility of focal cryoablation (Figure 1-A) for PV isolation was initially studied in 52 patients with paroxysmal and persistent AF who underwent PV isolation using this approach.[18] While 97% of the targeted PVs were successfully isolated, freedom from AF was only 56% at 1 year. Though in this study the longterm clinical efficacy appeared to be lower than conventional RF ablation, post-procedural computed tomographic (CT) surveillance demonstrated no evidence of PV stenosis. Hoyt et al.[19] also reported on the feasibility of focal cryoablation in a cohort of 31 paroxysmal AF patients. Acute PV isolation was attainable in 94% of patients, but freedom from AF was only 58% at 6 months. Once again, no cases of PV stenosis were encountered on serial CT surveillance. Similarly, Kenigsberg et al.[20] found that in fact focal cryoablation up to 15 mm inside the PV ostium was not associated with increased risk of PV stenosis. Furthermore, endoscopic studies have reported lack of esophageal ulcerations following focal cryoablation as compared with the cryoballoon or RF.[21] Nonetheless, the practical application of focal cryoablation for PV isolation appears limited by prolonged ablation times and reduced long-term efficacy. Additionally, there are no data currently on the clinical efficacy of linear focal cryoablation within the left atrium. While clinical studies in patients with atrial flutter (AFL) have shown that linear lesions can be effectively created by point-by-point cryoablation, long-term recovery of cavotricuspid isthmus conduction is generally higher using the latter approach as compared to RF.[22] Recently, the feasibility of a novel cryoablation system designed for catheter ablation of AF/AFL using a liquid refrigerant in place of nitrous oxide (used traditionally in catheterbased cryoablation systems), was described in vivo.[23] The latter is capable of achieving lower nadir temperatures and seems to hold promise for both PV isolation and linear ablations.
Figure 1. Cryoablation tools originally used for catheter ablation of AF. Panel A, shown, are a 6-mm and a 4-mm tip focal cryoablation catheter. Panel B, illustrates a curvilinear cryoablation catheter with a 64- mm freezing segment and the ability to expand to a diameter of 18 to 30 mm. This was the first cryoablation catheter specifically designed for PV isloation. Panel C, illustrates the ‘block the vein’ strategy – through this approach the PV is mechanically occluded using an angioplasty balloon catheter, advanced through the curvilinear cryoablation catheter to diminish PV blood flow and to further enhance the efficacy of cryoablation.
Cryoballoon Ablation Of AF
In order to overcome the challenges associated with focal cryoablation for PV isolation, a curvilinear catheter was initially developed in early 2000s. This catheter consisted of a 64-mm freezing segment with the ability to expand to a diameter from 18 to 30 mm (Figure 1-B). Skanes et al.[24] reported on the use of this circular cryoablation catheter. Although using this ablation tool, complete PV isolation proved possible in 91% of patients without any cases of PV stenosis, only 22% exhibited freedom from AF at 6 months. On the other hand, in 44% of patients who underwent a repeat procedure, PV reconnection was evident in 93% of the previously isolated PVs. The poor efficacy associated with this catheter was attributed largely to the undesirable effects of PV blood flow on cryoablation using this technology and its suboptimal catheter design. As a result, the ‘block the vein’ strategy was proposed (Figure 1-C). Eventually, based on this scheme, the first cryoballoon ablation catheter was introduced and subsequently tested in vivo.[25,26]
First-Generation (Arctic Front) Cryoballoon
The cryoballoon (Arctic Front, Medtronic, Inc, Minneapolis, MN) is a steerable, over the wire, 12-French double-walled balloon catheter system (Figure 2-A). Two sizes are available – a 23 and a 28 mm balloon catheter. Early on, a small study comparing the outcomes between the curvilinear cryoablation catheter and the cryoballoon pointed to the superior efficacy associated with the use of the latter in patients with paroxysmal AF.[27] Subsequently, acute and long-term safety and efficacy of PV isolation using the cryoballoon was evaluated in several non-randomized studies in patients with paroxysmal AF (Table 1), reporting long-term success rates ranging between 55 and 86%.[28–37] Neumann et al.[29] reported on a prospective, 3-center experience of cryoballoon ablation in 346 patients with symptomatic, drug refractory paroxysmal and persistent AF. Acute PV isolation could be achieved in 97% of the targeted PVs, and freedom from AF was 74% in patients with paroxysmal and 42% in those with persistent AF. No PV stenosis was again encountered during follow-up. However, transient phrenic nerve (PN) palsy occurred in 7.5% of patients; though they all resolved within 1 year. The Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) trial is the only published multicenter, prospective, randomizedcontrolled trial that evaluated the safety and efficacy of cryoballoon ablation for treatment of AF.[8] In this study, 245 patients with paroxysmal (78%) or early persistent AF (22%) were randomized to cryoballoon ablation or AAD therapy in a 2:1 randomization scheme. Cryoablation achieved electrical isolation in 98.2% of PVs, in 97.6% of patients. Following a 3-month blanking period, freedom from AF was achieved in 69.9% of patients treated with cryoablation as compared to only 7.3% using AAD therapy (p < 0.001). Transient PN palsy was encountered in 11.2% which ultimately persisted in 1.5% of patients at 1 year. Stroke occurred in 2.2% and PV stenosis (defined as a reduction of >75% in cross-sectional area or a 50% reduction in PV diameter) in 3.1% of patients treated with cryoablation. Two patients with PV stenosis were symptomatic and one required PV stenting. Subsequently, a systematic review of 23 studies published on the outcomes of cryoballoon ablation among 1,308 patients with paroxysmal and persistent AF showed a 97.5% acute procedural success (PV isolation) with freedom from AF in 72.8% at 1 year.[38] These findings were generally consistent with those reported in STOP AF. More recently, Yorgun et al.[39] reported on the additional benefits of cryoballoon-based ablation of AF beyond PV isolation. The authors found that modification of ganglionic plexi as evaluated by occurrence of vagal reactions during cryoablation may serve as an independent predictor of AF recurrence during long-term follow-up.
Figure 2. The designs of the first- and second-generation cryoballoon catheters, both advanced over an octapolar spiral mapping catheter specifically designed for recording PV potentials to guide real-time PV isolation. Panel A, in the first-generation cryoballoon, the maximal cooling zone (arrow) consists of an equatorial band. Accordingly, optimal balloon alignment would be vital to ensure proper circumferential contact between the PV antra and the cooling zone on this balloon. Panel B, due to design modifications made to the second-generation cryoballoon, the maximal cooling zone on this catheter (arrow) now spans the entire distal half of its surface including the distal tip. This in turn offers a greater cooling surface area while minimizing the impact of balloon orientation on optimal tissue contact.
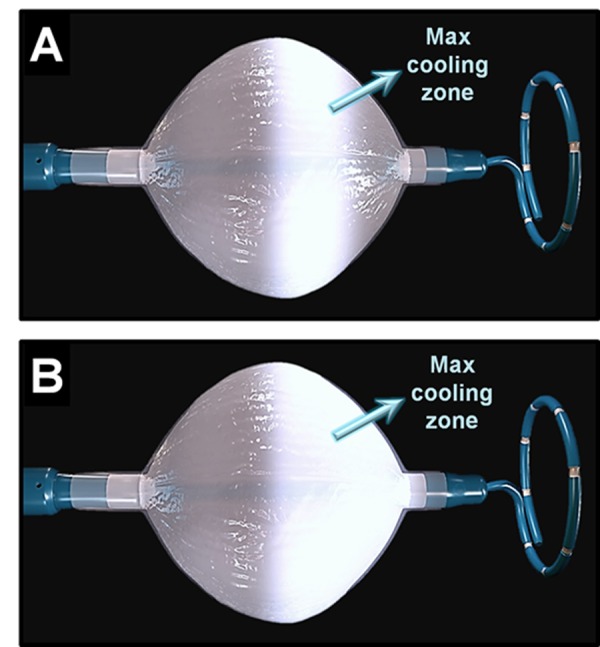
Table 1. Acute and long-term efficacy and safety of PV isolation using the first-generation cryoballoon in non-randomized studies.
| Study | n | Paroxysmal AF, n | Acute PV isolation | Procedure time, min | Fluoroscopy time, min | Transient PN palsy | Persistent PN palsy | Freedom from AF during follow-up |
|---|---|---|---|---|---|---|---|---|
| Malmborg, et al.[28] | 43 | 34 (79%) | 91% | 239 ± 48 | 57 ± 21 | 4.7% | 2.3% | 52% at 9 months |
| Neumann, et al.[29] | 346 | 293 (85%) | 97% | 170 | 40 | 7.5% | 0% | 74% at 12 months (paroxysmal AF) 42% at 12 months (persistent AF) |
| Klein, et al.[30] | 21 | 21 (100%) | 95% | 165 ± 35 | 39 ± 9 | 14.3% | 4.8% | 86% at 6 months |
| Van Belle, et al.[31] | 141 | 141 (100%) | 99% | 207 ± 79 | 50 ± 28 | 2.8% | 0% | 55% at 15 months |
| Chun, et al.[32] | 27 | 27 (100%) | 98% (single application) | 220 | 50 | 11.1% | 0% | 70% at 9 months |
| Defaye, et al.[33] | 117 | 92 (79%) | 87% (single application) | 155 ± 43 | 35 ± 15 | 0.9% | 0% | 69% at 12 months (paroxysmal AF) 45% at 12 months (persistent AF) |
| Vogt, et al.[34] | 605 | 579 (96%) | 91% | 156 | 25 | 2% | 0% | 62% at 30 months |
| Ferrero-de Loma- Osorio, et al.[35] | 63 | 40 (63%) | 95% | 180 ± 32 | 31 ± 22 | 4.8% | 0% | 72% at 2 years (paroxysmal AF) 36% at 2 years (persistent AF) |
| Aytemir, et al.[36] | 236 | 188 (80%) | 99% | 72 ± 5 | 14 ± 3 | 4.6% | 0% | 81% at 18 months (paroxysmal AF) 50% at 18 months (persistent AF) |
| Rao, et al.[37] | 51 | 51 (100%) | 97% | 151 ± 30 | 49 ± 12 | 5.9% | 0% | 57% at 36 months |
Second-Generation (Arctic Front Advance) Cryoballoon
Soon after the early experiences with the first-generation cryoballoon it became apparent that ablation using this tool was prone to certain challenges and drawbacks. Due to its number and location of refrigerant injection ports, the maximal cooling zone on the first-generation balloon occurs primarily over its equator (Figure 2-A). Therefore, optimal balloon positioning and orientation at PV antra is often critical when using this catheter, such that balloon mal-alignment can frequently compromise uniform tissue cooling and lesion formation.[40] This is further supported by more recent data showing that durable PV isolation is directly impacted by the degree of PV occlusion and tissue cooling, which in turn is influenced by the distance from the balloon (cooling zone These concerns subsequently led to the development of the second-generation cryoballoon (Arctic Front Advance, Medtronic, Inc). The principal modification in the design of this catheter has to do with the expansion of the cooling zone to the entire distal half of its surface (Figure 2-B). Knecht et al,[42] analyzed the magnitude of ice formation using this new design as compared to the first-generation cryoballoon and found that the mean covered surface areas were significantly different for the 28-mm but not the 23-mm balloons. Where as the first-generation catheter created non-contiguous ice formation, the second-generation cryoballoon exhibited a rather homogenous ice cap covering the entire distal segment of the balloon including its distal pole (the nose of the balloon). The superior efficacy of the second-generation cryoballoon was subsequently validated in vivo.[43,44] That is, it was shown that cryoablation of canine PVs through a single 4-min cryoapplication using the second-generation cryoballoon created transmural and circumferential lesions resulting in electrical isolation in 100% of PVs, as compared to only 60% using the first-generation cryoballoon. A more recent clinical study showed that cryoablation using this balloon was wide and circumferential with the level of PV isolation more antral, resulting in generous posterior left atrial debulking which could in part also account for this balloon’s improved efficacy.[45] Furthermore, Reddy et al.[46] evaluated the outcomes of PV isolation using the second-generation cryoballoon in 21 consecutive patients with paroxysmal AF, all of whom subsequently underwent a second remapping procedure to assess for durability of PV isolation at 3 months. The authors found that acute electrical isolation could be achieved in 83% of PVs using a single cryoapplication, with 91% of PVs still durably isolated at 3 months. This provides clinical evidence that in fact the improved thermodynamic characteristics of the second-generation cryoballoon seem to be associated with a higher rate of both single-shot PV isolation and also chronic lesion durability, which may translate into improved clinical outcomes.
Meanwhile, several studies have compared the acute and long-term outcomes of PV isolation using the first- and second-generation cryoballoons in patients with paroxysmal and persistent AF.[47–53] These studies collectively point to the superiority of the second- over the first-generation cryoballoon based upon several major benchmark parameters including acute PV isolation, biophysical characteristics, ablation time, procedure time, fluoroscopic utilization, and longterm freedom from AF (Table 2). As previously shown by our group, in addition to faster balloon cooling rates at 30 and 60 sec, shorter time-to-nadir temperature, and longer interval and total thaw times observed with the use of the second-generation cryoballoon, we also found a significantly lower PV reconnection rate at repeat procedure in those with arrhythmia recurrence during long-term followup (30% versus 13%; p=0.037).[49] Furthermore, these results were independent of operator experience and learning curve. Furthermore, Bordignon et al.[54] evaluated the magnitude of biomarker release in 66 patients following cryoablation using the first- versus the secondgeneration cryoballoons and found that despite shorter ablations required using the second-generation cryoballoon, higher levels of cardiac biomarkers such as troponin T, creatine phosphokinase and lactate dehydrogenase could be detected within the first 48 h in patients treated with the latter – possibly suggestive of more effective ablation. Interestingly, these findings also correlated with higher procedural success at 6 months. Table 3, illustrates a summary of mid- and long-term clinical outcomes of AF ablation using the second-generation cryoballoon as assessed by non-randomized single and multi-center studies.[46–58] These studies have consistently reported improved procedural and clinical outcomes associated with the use of the second-generation cryoballoon.
Table 2. Acute and long-term outcomes of PV isolation using the first- versus second-generation cryoballoons in non-randomized studies.
L*Cryoballoon temperature at PV isolation, **Time to PV isolation, †Nadir cryoballoon temperature, ‡Cryoballoon temperature at 60 sec, §Time to nadir temperature, ¶Cryoballoon temperature at PV, isolation using the 23-mm cryoballoon, #Cryoballoon temperature at PV isolation using the 28-mm cryoballoon, ††Time to PV isolation using the 23-mm cryoballoon, ‡‡Time to PV isolation using the 28-mm cryoballoon
| Study | n | Acute PV isolation | Cryoballoon temperature at PV isolation, at 60 sec or, at nadir temperature, ºC | Time to PV isolation or nadir temperature, sec | Ablation Time, min | Procedure time, min | Fluoroscopy time, min | Transient PN palsy | Freedom from AF at 1 year |
|---|---|---|---|---|---|---|---|---|---|
| Martins, et al.[47] First-generation balloon Second-generation balloon p-value | 66 81 | 81% 90% 0.003 | –36 ± 10* –32 ± 10* 0.001 | 52 ± 34** 40 ± 25** 0.001 | 26 ± 14 22 ± 7 <0.001 | 120 ± 24 107 ± 24 0.002 | 29 ± 10 25 ± 9 0.020 | 10.6% 24.4% 0.048 | N/A N/A N/A |
| Fürnkranz, et al.[48] First-generation balloon Second-generation balloon p-value | 30 30 | 100% 100% 1.00 | –49 ± 6† –52 ± 6† 0.005 | 79 ± 60** 52 ± 36** 0.049 | N/A N/A N/A | 128 ± 27 98 ± 30 <0.001 | 19 ± 7 13 ± 5 0.001 | 3.3% 3.3% 1.00 | N/A N/A N/A |
| Aryana, et al.[49] First-generation balloon Second-generation balloon p-value | 140 200 | 92% 98% 0.036 | 26 ± 23‡ –32 ± 16‡ <0.001 | 232 ± 77§ 209 ± 68§ <0.001 | 61 ± 17 47 ± 12 <0.001 | 209 ± 58 154 ± 47 <0.001 | 42 ± 17 27 ± 12 <0.001 | 12.1% 16.0% 0.311 | 80% 84% 0.289 |
| Straube, et al.[50] First-generation balloon Second-generation balloon p-value | 364 120 | 99% 100% 0.43 | 61¶ / −50# −58¶ / −52# <0.001¶ / 0.074# | 48†† / 76‡‡ 33†† / 52‡‡ <0.001†† / <0.001‡‡ | 60 ± 16 58 ± 12 0.007 | 185 ± 49 175 ± 45 0.038 | 34 ± 12 29 ± 11 <0.001 | 20.6% 27.5% 0.121 | 85% 85% 1.00 |
| Fürnkranz, et al.[51] First-generation balloon Second-generation balloon p-value | 50 55 | 98% 100% 0.48 | N/A N/A N/A | N/A N/A N/A | 52 ± 10 33 ± 6 <0.001 | 137 ± 33 94 ± 24 <0.001 | 22 ± 10 13 ± 4 <0.001 | 8.0% 12.7% 0.434 | 64% 84% 0.008 |
| Di Giovanni, et al.[52] First-generation balloon Second-generation balloon p-value | 50 50 | 100% 100% 1.00 | –50 ± 10† –52 ± 5† --- | 69 ± 25** 43 ± 17** <0.05 | N/A N/A N/A | 115 ± 39 90 ± 16 <0.01 | 25 ± 6 18 ± 6 <0.01 | 8.0% 16.0% 0.218 | 66% 84% 0.038 |
| Liu, et al.[53] First-generation balloon Second-generation balloon p-value | 57 68 | 88% 93% 0.352 | –42 ± 6† –46 ± 6† 0.003 | N/A N/A N/A | 37 ± 10 28 ± 9 <0.001 | 117 ± 26 103 ± 23 0.001 | 20 ± 5 18 ± 5 0.011 | 0% 2.9% 0.159 | 60% 90% <0.001 |
Table 3. Acute and long-term efficacy and safety of PV isolation using the second-generation cryoballoon in non-randomized studies.
| Study | n | Paroxysmal AF, n | Acute PV isolation | Ablation time, min | Procedure time, min | Fluoroscopy time, min | Transient PN palsy | Persistent PN palsy | Freedom from AF during follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Kenigsberg, et al.[46] | 43 | 34 (79%) | 100% | 22 ± 4 | 126 ± 23 | 16 ± 8 | N/A | N/A | 95% at 6 months |
| Martins, et al.[47] | 81 | 81 (100%) | 90% | 22 ± 7 | 107 ± 24 | 25 ± 9 | 3.3% | 0% | N/A |
| Fürnkranz, et al.[48] | 30 | 23 (77%) | 100% | 29 ± 12 | 98 ± 30 | 13 ± 5 | 3.3% | 0% | N/A |
| Aryana, et al.[49] | 200 | 143 (72 %) | 98% | 47 ± 12 | 154 ± 47 | 27 ± 12 | 16.0% | 0.5% | 84% at 1 year |
| Straube, et al.[50] | 120 | 63 (52%) | 100% | 58 ± 12 | 175 ± 45 | 29 ± 11 | 27.5% | 1.7% | 85% at 1 year |
| Fürnkranz, et al.[51] | 55 | 55 (100%) | 100% | 33 ± 6 | 94 ± 24 | 13 ± 4 | 12.7% | 5.4% | 84% at 1 year |
| Di Giovanni, et al.[52] | 50 | 50 (100%) | 100% | N/A | 90 ± 16 | 18 ± 6 | 16.0% | 2% | 84% at 1 year |
| Liu, et al.[53] | 68 | 50 (74%) | 93% | 28 ± 9 | 103 ± 23 | 18 ± 5 | 2.9% | 0% | 90% at 1 year |
| Bordignon et al.[54] | 33 | 26 (79%) | 100% | 33 ± 6 | N/A | N/A | 6.1% | 0% | 85% at 6 months |
| Chierchia, et al.[55] | 42 | 42 (100%) | 100% | 31 ± 4 | 95 ± 16 | 20 ± 12 | 19.0% | 0% | 83% at 1 year |
| Metzner, et al.[56] | 50 | 36 (72%) | 100% | 35 ± 6 | 140 ± 28 | 25 ± 8 | 2.0% | 0% | 80% at 1 year |
| Ciconte, et al.[57] | 143 | 113 (79%) | 100% | 13 ± 5 | 95 ± 16 | 13 ± 8 | 6.3% | 3.5% | 80% at 1 year |
| Aryana, et al.[58] | 633 | 472 (75%) | 98% | 40 ± 14 | 145 ± 49 | 29 ± 13 | 7.6% | 1.1% | 77% at 1 year |
PV Isolation Using The Cryoballoon And Predictors Of AF Recurrence
A more recent feature of cryoballoon ablation of AF is the ability to potentially monitor real-time to PV isolation, also known as timeto- effect via a single transseptal access. That is, the cryoballoon can be advanced into the left atrium either over a conventional guide wire or a specific octapolar spiral mapping catheter/guide wire (Achieve, Medtronic, Inc) designed for monitoring and recording of PV potentials to guide real-time PV isolation (Figure 2). To date, this approach has been validated in several studies.[59–61] Nonetheless, the main limitation of this catheter has to do with its smaller diameter size (either 15-mm or 20-mm), precluding consistent real-time recording of PV potentials in patients with larger PV antra. Additionally, the wider spacing among the electrodes on the catheter further amplifies far-field electrogram sensing. In a recent publication, Boveda et al[61] reported a stepwise approach using this catheter which could accurately assess real-time PV isolation in ~98% of the PVs. Though in our experience, we have been unable to duplicate such a high rate of confirmation of PV isolation during cryoablation using this spiral recording catheter, undoubtedly this remains a highly effective tool for measuring time-to-effect providing overall a simpler method for validation of PV isolation as compared to other single-shot ablation systems such as the nMARQ or the pulmonary vein ablation catheter (PVAC).
Meanwhile several other studies have closely examined the predictors of a successful cryoballoon ablation of AF. One report found an inverse association between the ovality index and the orientation of PV ostia as determined by cardiac CT angiography with the degree of cryoballoon occlusion during catheter ablation. [62] Similarly, Kubala et al.[63] found that in patients undergoing cryoballoon ablation, presence of normal versus atypical PV anatomy such as a common left PV, was associated with improved freedom from AF during long-term follow-up. With respect to biophysical characteristics of cryoballoon ablation, it seems that balloon thaw time and perhaps its secondary derivative, freeze area-under-thecurve, represent significant predictors of PV reconnection during follow-up and long-term freedom from AF post-catheter ablation.[64,65] On the other hand, cryoablation time and cryoballoon temperature served as poor and unreliable predictors of such endpoints.[65] It should be emphasized that the freeze area-under-the-curve signifies a comprehensive metric to assess the magnitude of cryoablation.[49] As such, the computed value collectively reflects a multitude of parameters including duration of cryoapplication, rate of cooling, nadir temperature, and thaw-time. Meanwhile, another study has suggested that very cold minimum balloon temperatures (<-51°C) may in fact be predictive of acute PV isolation.[66] Conversely, the same study found that a minimum balloon temperature ≥-36°C (for superior PVs) and ≥-33°C (for inferior PVs) predicted failed acute PV isolation with a relatively high specificity (≥95%). But it should be pointed out that in this study no data on long-term outcomes were reported to further corroborate the acute procedural findings with respect to durability of PV isolation or freedom from AF. Collectively, we believe that these findings underscore the importance of the ‘quality’ as opposed to the ‘quantity’ of cryoapplications during catheter of ablation when using the cryoballoon.
In the meantime, there remains a lack of consensus on the appropriate ablation dosing when performing an AF ablation using the cryoballoon – that is, with respect to the ideal freezing duration and the number of freeze-thaw-freeze cycles. The current recommendations suggest a 4-min cryoapplication along with a ‘double freeze’ approach (freeze-thaw-freeze cycle). Though the ‘double freeze’ method has been shown to result in more extensive tissue destruction and deeper, larger lesions due to the repeated freeze/thaw effects on the cell membrane,[14] it has been argued that this data may largely pertain to the less potent cryoablation tools such as the focal cryoablation catheter. Indeed, there is cumulative evidence in support of improved acute and long-term efficacy associated with a single PV cryoapplication using the second-generation cryoballoon. Ciconte et al.[57] recently reported their results of a single 3-min cryoapplication using the second-generation cryoballoon in 143 consecutive patients. The authors achieved acute PV isolation in 94% of PVs using a single application and in 100% after 1.1 ± 0.4 freezes. After a 3-month blanking period, freedom from atrial arrhythmias was achieved in 80% of patients at 1 year (82% with paroxysmal versus 73% with persistent AF). Additionally, 10% of patients underwent a repeat procedure. Though this data is subject to selection bias, among these patients 43% of PVs exhibited conduction recovery at redo ablation. Now it should be called to attention that it would be extremely difficult to meaningfully compare such data against those derived from other non-matched series. However, as previously reported by our group,[49] the same outcome of PV reconnection in patients undergoing repeat procedures following an initial second-generation cryoballoon ablation using ≥2 applications (≥1 freeze-thaw-freeze cycle) was found to be 13%. In the long run, whether a second ‘bonus’ freeze will in fact prove necessary still needs to be determined.
A recent study has also evaluated the predictive value of early AF recurrence following catheter ablation using the first-generation cryoballoon by analyzing data from the STOP AF trial.[67] The authors found that over half of the patients (51%) experienced an early recurrence post-ablation within the first 3 months. Moreover, of these recurrences the great majority (85%) had occurred within the first month. Though nearly half of these individuals (44%) remained free of long-term atrial arrhythmias, early recurrence did in fact correlate with late recurrence of AF. Conversely, only 13% of those with early recurrences were found to have recurrent AF during longterm follow-up.
Comparison Of Cryoballoon Versus RF
Though there is limited data on prospective head-to-head comparisons between cryoballoon versus RF catheter ablation of AF, several non-randomized comparative studies[58,68–76] have been published on the use of the first-generation cryoballoon as compared to open-irrigated, non-force sensing RF (Table 4). The results have been largely mixed without any apparent, significant differences between the two modalities. However, two of the larger series by Kojodjojo et al.[69] and Mugnai et al.[75] did in fact illustrate subtle trends towards improved 1-year outcomes with the first-generation cryoballoon as compared to RF (77% versus 72% and 63% versus 57%, respectively). Xu et al.[77] reported the outcomes from a metaanalysis of 1,104 patients from published studies, who underwent AF ablation using the cryoballoon (n=469) or RF (n=635). They found cryoablation to be associated with a significantly shorter procedure time (by a weighted mean of 30 min) and fluoroscopy exposure (by a weighted mean of 14 min), whereas ablation time was nonsignificantly longer with cryoablation (by a weighted mean of 12 min). Moreover, cryoablation was also found to be associated with a non-significantly higher rate of long-term success as compared with RF. Recently, our group has reported on the acute and longterm outcomes from a large, non-randomized, multicenter study comparing the second-generation cryoballoon to open-irrigated, non-force sensing RF.[58] The study included 1,196 patients with AF (76% paroxysmal), and it found that cryoablation was associated with a superior primary endpoint of freedom from atrial arrhythmias at 12 months following a single catheter ablation procedure without the use of AAD therapy (76.6% versus 60.4%; p<0.001), and overall a reduced need for AADs (16.7% versus 22.0%; p=0.024) and fewer repeat ablations (14.6% versus 24.1%; p<0.001) , as compared to non-contact force sensing RF. In addition, at redo procedure, fewer patients exhibited PV reconnection if previously ablated using the cryoballoon (44.2%) as versus RF (65.7%); p=0.002. Cryoablation was also associated with shorter ablation and procedure times, but greater fluoroscopic utilization. Both transient and persistent PN palsy occurred exclusively with cryoablation, whereas all other adverse event rates were similar between the two groups. These findings coupled with the relative safety associated with the use of cryoablation using the second-generation cryoballoon, reproducibility of the results across a number of different centers with variable procedural volume, and suggestion of a similar magnitude of benefit in patients with both paroxysmal and persistent AF, evoked the second-generation cryoballoon a more favorable ablation tool as compared to non-force sensing RF with an AF ablation score[78] notably greater than that computed for RF. Additional investigations to evaluate the safety and efficacy related to the use of cryoballoon ablation in comparison to the recently made available force-sensing RF ablation catheters seems necessary to identify the most optimal approach to AF ablation. Along these lines, Jourda et al.[79] reported on a prospective comparison between force sensing RF and the second-generation cryoballoon. The study found that both procedural and fluoroscopic times were shorter with force sensing RF but with similar ablation times, adverse events, and long-term freedom from AF as compared to cryoablation using the second-generation balloon. At this point, a larger, multicenter comparison of these two diverse types of ablation techniques with respect to cost, safety, and efficacy seems relevant.
Table 4. Acute and long-term outcomes of PV isolation using RF versus the cryoballoon in non-randomized studies.
*Combination of first- and second-generation cryoballoons, **Second-generation cryoballoon only, †Force sensing RF
| Study | n | Paroxysmal AF | Acute PV isolation | Ablation Time, min | Procedure time, min | Fluoroscopy time, min | PN palsy | Other adverse events | Freedom from AF during long-term follow-up |
|---|---|---|---|---|---|---|---|---|---|
| Linhart, et al.[68] RF Cryoballoon | 20 20 | 20 (100%) 20 (100%) | 100% 81% | N/A N/A | 200 ± 67 166 ± 39 | 55 ± 23 41 ± 13 | 0% 15% | 0% 0% | 45% at 6 months 50% at 6 months |
| Kojodjojo, et al.[69] RF Cryoballoon | 53 90 | 53 (100%) 90 (100%) | 99% 83% | N/A N/A | 208 ± 58 108 ± 28 | 62 ± 36 27 ± 9 | 0% 2.2% | 3.8% 1.1% | 72% at 1 year 77% at 1 year |
| Tayebjee, et al.[70] RF Cryoballoon | 25 25 | 25 (100%) 25 (100%) | 100% 76% | N/A N/A | 35 45 | 0% 8% | 0% 4% | 4% 4% | 52% at 1 year 56% at 1 year |
| Kühne, et al.[71] RF Cryoballoon | 25 25 | 25 (100%) 25 (100%) | 00% 100% | 47 45 | 197 ± 52 166 ± 32 | 46 ± 22 61 ± 25 | 0% 4% | 4% 4% | 92% at 1 year 88% at 1 year |
| Sorgente, et al.[72] RF Cryoballoon | 29 30 | 20 (69%) 24 (80%) | 100% 100% | N/A N/A | N/A N/A | N/A N/A | 0% 10% | 13.8% 3.3% | 66% at 1 year 66% at 1 year |
| Herrera Siklódy, et al.[73] RF Cryoballoon | 30 30 | 17 (57%) 21 (70%) | 100% 100% | 52 ± 21 44 ±6 | 200 ± 46 177 ± 30 | 37 ± 16 38 ± 12 | 0% 6.7% | 0% 6.7% | 80% at 1 year 63% at 1 year |
| Schmidt, et al.[74] RF Cryoballoon | 2,870 905 | 2,870 (100%) 905 (100%) | 98% 97% | 33 45 | 165 160 | 24 34 | 0% 2.1% | 4.6% 2.7% | N/A N/A |
| Mugnai, et al.[75] RF Cryoballoon | 260 136 | 260 (100%) 136 (100%) | 100% 100% | 43 ± 6 45 ± 4 | 192 ± 49 112 ± 58 | 36 ± 14 31 ± 17 | 0% 8.1% | 14.2% 11.0% | 57% at 23 months 63% at 23 months |
| Juliá, et al.[76] RF† Cryoballoon* | 186 100 | 186 (100%) 100 (100%) | 98% 100% | N/A N/A | 190 ± 57 117 ± 59 | 35 ± 19 27 ± 16 | N/A N/A | N/A N/A | 80% at 1 year 81% at 1 year |
| Aryana, et al.[58] RF Cryoballoon** | 422 633 | 319 (76%) 472 (75%) | 99% 98% | 66 ± 26 40 ± 14 | 188 ± 42 145 ± 49 | 23 ± 14 29 ± 1 | 0% 7.6% | 2.6% 1.6% | 60% at 1 year 77% at 1 year |
| Jourda, et al.[79] RF† Cryoballoon** | 75 75 | 75 (100%) 75 (100%) | 100% 100% | 2 ± 13 32 ± 3 | 111 ± 32 134 ± 48 | 21 ± 8 25 ± 10 | 0% 17.3% | 2.7% 1.3% | 88% at 1 year 85% at 1 year |
In addition, Juliá and colleagues[76] recently reported on the incidence and mechanism of atrial tachycardias following catheter ablation of paroxysmal AF in 286 consecutive patients using the cryoballoon versus RF. The authors found that the incidence of postablation atrial tachycardias was significantly lower with cryoablation as compared to RF (3.0% versus 11.3%; p= 0.028). This difference was driven largely by the larger (28-mm) second-generation cryoballoon. Though not entirely clear, the mechanism is believed to be related to the overall reduced atrial ablation and perhaps larger and more homogeneous lesions created using the latter balloon.
Cryoballoon Ablation Of Persistent AF
Since the STOP AF study which originally evaluated the outcomes of cryoablation in patients with paroxysmal and ‘early’ persistent AF, several non-randomized first-/second-generation cryoballoon studies have evaluated this therapy in those with both paroxysmal and persistent AF.[46,48–50,53,54,56,58] Specifically, a few additional studies have explicitly examined the efficacy of cryoablation of non-PV triggers using the second-generation cryoballoon.[80–82] In a recent multicenter study, the second-generation cryoballoon was shown to be a safe and effective tool for electrical isolation of the superior vena cava and ablation of the left atrial roof, the left lateral ridge and the base of the left atrial appendage throughout both atria in 110 patients with persistent and long-standing persistent AF.[82] Complications were rare and at 1 year, 78% of patients remained free of AF recurrence following a 3-months blanking period. Obviously, additional data is currently needed to further validate the acute and long-term outcomes using this approach.
‘Hybrid’ Approach
Recently, a ‘hybrid’ approach involving a thoracoscopic surgical and a concomitant endocardial cryoballoon PV ablation has been described in patients with persistent AF or in those with paroxysmal AF and a failed prior catheter ablation.[83] While in two small studies this approach proved safe and feasible, the long-term efficacy of this strategy has yet to be evaluated.[83,84] For now, the precise role, applicability and specific advantages of the above-mentioned approach over each of the individual strategies alone, remain unclear.
Safety
Several studies have established the overall safety of cryoballoon ablation of AF,[8,38,85] While some have suggested fewer major adverse events associated with the use of cryoballoon versus RF including fewer cardiac perforations and fatalities,[85] these observations have not been entirely consistent. Aside from PN palsy which remains the most frequent complication related to the use of cryoballoon,[8,85] the same adverse events that in general complicate RF ablation also occur with cryoablation of AF.[38,58] These consist of groin complications, bleeding, thromboembolism, pericardial effusion, gastroparesis, and atrioesophageal fistula.[8,38,85] Though thromboembolism remains rare in the setting of cryoablation,[15] most embolic events as a consequence of cryoablation are believed to represent air embolism related to the handling of the larger sheath inside the left atrium. Neumann et al.86 investigated the incidence of micro-embolization immediately after catheter ablation of AF with the cryoballoon versus RF in 89 patients using cerebral magnetic resonance. The authors discovered presence of asymptomatic cerebral lesions one day post-ablation in 8.9% of patients ablated with cryoballoon versus 6.8% with RF. These outcomes did not differ statistically. Meanwhile, PV stenosis may also complicate cryoablation of AF. Thought this adverse event has historically been thought to be a rare sequela of cryoablation,[14] there is sufficient evidence to suggest that cryoballoon ablation is not immune to this type of complication.[8] Nonetheless, a recent meta-analysis documented the overall incidence of PV stenosis resulting in symptoms or requiring intervention at only 0.17% in patients who underwent cryoballoon ablation of AF.[38] Chierchia et al. investigated the incidence and outcomes of pericardial effusion following cryoballoon versus RF ablation, and found no significant difference between the two modalities (11% versus 16%).[87] The authors concluded that this complication was generally asymptomatic and mild with a benign self-limiting course in nearly all cases. Lastly, persistent iatrogenic atrial septal defect (iASD) following cryoablation has also been described.[88–90] Specific concerns surrounding this complication have been raised due to the use of the larger, 15-French transseptal sheath which is required for delivery of the cryoballoon catheter into the left atrium. The incidence of iASD in cryoablation studies varies between 16–31% during short-term follow-up[88,89] and has been reported as high as 20% at 1 year.[90] Not surprisingly, this incidence seems to be higher than that which is reported for RF which generally utilizes a smaller transseptal sheath for performing the ablation.[89] Though most patients with persistent iASD seem to tolerate this entity rather well and without apparent adverse events,[88–90] additional studies on larger patient populations with longer follow-up are needed to reach a firm conclusion.
Meanwhile, some of the more important and specific adverse events complicating cryoballoon ablation of AF are reviewed in the ensuing sections.
PN Palsy
PN palsy is by far the most common complication of cryoballoon ablation of AF.[8,38] Anatomical studies have revealed the close proximity of the right PN to the superior vena cava and the anteriorinferior aspect of the right superior PV, and also the left PN to the left atrial appendage.[78] Hence, catheter ablation in the vicinity of these structures can potentially yield collateral injury to the adjacent PN. However, PN injury is not unique to cryoablation. In fact, it can also occur as a consequence of catheter ablation using RF as well as other energy modalities.[91,92] Overall, the prevalence of PN palsy due to AF ablation is estimated between 0.37% and 1.6%.[91] A recent study suggested that the mechanism of PN injury as a result of cryoablation seems to be axonal in nature and characterized by Wallerian degeneration, with great potential for regeneration and neuronal recovery.[93] Consistent with this, the short-term outcome of patients with post-ablation PN palsy appears to be favorable with >80% achieving complete resolution by 1 year.[91] As such, PN palsy may be classified as either transient or persistent. While the incidence of transient right PN palsy as a consequence of cryoballoon ablation of AF can reach ≈20%, persistent PN palsy remains uncommon with a reported incidence of only 0–4% in most studies.[29,31,34,36,49,50,58,74,75] Moreover, transient but not persistent right PN palsy has been shown to occur more frequently using the second-generation as compared to the first-generation cryoballoon.[47,49,52,94] This is likely due to the second-generation catheter’s increased potency. Furthermore, PN palsy has also been shown to occur more frequently with the use of the 23-mm cryoballoon.[44,94] In most cases, the latter is believed to be related to the deployment of a relatively undersized cryoballoon deeper inside the PV.[95] In addition to minimizing the physical distance between the cryoballoon and the PN, cryoablation at a relatively more distal position inside the right PVs may be more conducive to enhanced ‘cold’ transfer to deeper tissues such as the PN due to reduced convective heating of the balloon by atrial blood flow.[94–96] Hence, this may result in deeper penetration of cryoenergy, thereby increasing the risk of collateral injury.
Esophageal Injury
Esophageal thermal injury seems to occur with left atrial ablation virtually using any type of energy modality,[97,98] including also cryoenergy.[99–102] Furthermore, it is believed that in some patients this may represent a precursor to atrioesophageal fistula.[103] Early experiences using the first-generation cryoballoon suggested possibly a lack of esophageal thermal injury associated with cryoablation as assessed on post-procedural endoscopy, despite steep luminal esophageal temperature drops during catheter ablation.[104] Nonetheless, esophageal thermal injury and ulceration were subsequently demonstrated in other clinical studies,[105] particularly with the use of the second-generation cryoballoon which can be associated with esophageal ulcerations in as many as ≈20% of patients.[106,107] It should be emphasized that this incidence generally remains similar and possibly lower than that reported with RF.[108] Though as with ablation using other energy modalities no precise measures have been identified to mitigate esophageal thermal injury, Fürnkranz et al.[107] found that a luminal esophageal temperature ≤12ºC during cryoballoon ablation predicted esophageal ulceration with 100% sensitivity and 92% specificity. In a subsequent study,[111] these authors reported that by adopting a strategy of luminal esophageal temperature-guided cryoablation through interruption of ablation with esophageal temperatures ≤15°C, the esophageal ulceration rate decreased to 3%. As such, avoidance of ultra-cold luminal esophageal temperatures during cryoablation seems prudent.
Lung Injury
Both cough and hemoptysis have been reported following cryoablation of AF.[112–118] A persistent dry cough can be detected more commonly in some patients following cryoballoon ablation. However, hemoptysis remains rather uncommon with an incidence ranging between 0–2.1%.[114–116] Though hemoptysis can also occur in the setting of PV stenosis, most cases of hemoptysis following cryoablation do not seem to accompany such a complication.[112–118] Furthermore, most overt cases of hemoptysis seem to manifest within hours to days following cryoablation,[112–114,116] rendering the possibility of PV stenosis once again less likely. Instead, it has been postulated that transient interruption of vascular integrity, perhaps within the pulmonary capillary system due to cryoinjury, may serve as a possible culprit.[113,114,116] Accordingly, some investigators have attributed this to colder balloon temperatures (<55ºC) and deeper balloon positioning inside the PVs during cryoablation.[112–114,116,117] CT imaging of patients with hemoptysis frequently demonstrates presence of edema surrounding the PV tissue sometimes with erosion,[112] with[118] or without[113,114,117] luminal narrowing. Mucosal hyperemia and erosion can also be detected during bronchoscopy.[113,114] Some investigators have ascribed these findings to pulmonary infarction.[116] While this remains unclear based on the reports to date, both the clinical symptoms and findings appear to be self-limiting with a gradual resolution over time.[112–116] Furthermore, none of these cases have been associated with catastrophic complications such as formation of a fistula.
Conclusions
Over the past decade, cryoballoon ablation of AF has emerged as a practical, alternative strategy to point-by-point RF ablation. There seem to be several reasons for this. First, the acute and longterm safety and efficacy associated with cryoablation appear to be similar to RF, in patients with both paroxysmal and also persistent AF. Second, this technology also offers certain advantages over conventional RF ablation including a gentler learning curve and relative ease of use, shorter ablation and procedure times, and lack of need for costly electroanatomical mapping equipment commonly used with RF ablation. More recently, with the advent of the secondgeneration cryoballoon, the effectiveness of cryoablation has further improved remarkably. Given that results from several cryoablation studies strongly suggest a greatly improved efficacy associated with the use of the second-generation cryoballoon, a prospective headto- head comparison between the latter and force sensing RF seems appropriate. As such, we eagerly await the results of ongoing studies that are currently investigating this topic.
Disclosures
Drs. Aryana and O’Neill have received consulting fees, speaker honoraria and a research grant from Medtronic, Inc.
References
- 1.January Craig T, Wann L Samuel, Alpert Joseph S, Calkins Hugh, Cigarroa Joaquin E, Cleveland Joseph C, Conti Jamie B, Ellinor Patrick T, Ezekowitz Michael D, Field Michael E, Murray Katherine T, Sacco Ralph L, Stevenson William G, Tchou Patrick J, Tracy Cynthia M, Yancy Clyde W. 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. J. Am. Coll. Cardiol. 2014 Dec 2;64 (21):e1–76. doi: 10.1016/j.jacc.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 2.Wokhlu Anita, Monahan Kristi H, Hodge David O, Asirvatham Samuel J, Friedman Paul A, Munger Thomas M, Bradley David J, Bluhm Christine M, Haroldson Janis M, Packer Douglas L. Long-term quality of life after ablation of atrial fibrillation the impact of recurrence, symptom relief, and placebo effect. J. Am. Coll. Cardiol. 2010 May 25;55 (21):2308–16. doi: 10.1016/j.jacc.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 3.Piccini Jonathan P, Lopes Renato D, Kong Melissa H, Hasselblad Vic, Jackson Kevin, Al-Khatib Sana M. Pulmonary vein isolation for the maintenance of sinus rhythm in patients with atrial fibrillation: a meta-analysis of randomized, controlled trials. Circ Arrhythm Electrophysiol. 2009 Dec;2 (6):626–33. doi: 10.1161/CIRCEP.109.856633. [DOI] [PubMed] [Google Scholar]
- 4.Pappone C, Rosanio S, Oreto G, Tocchi M, Gugliotta F, Vicedomini G, Salvati A, Dicandia C, Mazzone P, Santinelli V, Gulletta S, Chierchia S. Circumferential radiofrequency ablation of pulmonary vein ostia: A new anatomic approach for curing atrial fibrillation. Circulation. 2000 Nov 21;102 (21):2619–28. doi: 10.1161/01.cir.102.21.2619. [DOI] [PubMed] [Google Scholar]
- 5.Wilber David J, Pappone Carlo, Neuzil Petr, De Paola Angelo, Marchlinski Frank, Natale Andrea, Macle Laurent, Daoud Emile G, Calkins Hugh, Hall Burr, Reddy Vivek, Augello Giuseppe, Reynolds Matthew R, Vinekar Chandan, Liu Christine Y, Berry Scott M, Berry Donald A. Comparison of antiarrhythmic drug therapy and radiofrequency catheter ablation in patients with paroxysmal atrial fibrillation: a randomized controlled trial. JAMA. 2010 Jan 27;303 (4):333–40. doi: 10.1001/jama.2009.2029. [DOI] [PubMed] [Google Scholar]
- 6.Oral Hakan, Pappone Carlo, Chugh Aman, Good Eric, Bogun Frank, Pelosi Frank, Bates Eric R, Lehmann Michael H, Vicedomini Gabriele, Augello Giuseppe, Agricola Eustachio, Sala Simone, Santinelli Vincenzo, Morady Fred. Circumferential pulmonary-vein ablation for chronic atrial fibrillation. N. Engl. J. Med. 2006 Mar 2;354 (9):934–41. doi: 10.1056/NEJMoa050955. [DOI] [PubMed] [Google Scholar]
- 7.Schmidt Boris, Metzner Andreas, Chun Kyoung Ryul Julian, Leftheriotis Dionysios, Yoshiga Yasuhiro, Fuernkranz Alexander, Neven Kars, Tilz Roland Richard, Wissner Erik, Ouyang Feifan, Kuck Karl-Heinz. Feasibility of circumferential pulmonary vein isolation using a novel endoscopic ablation system. Circ Arrhythm Electrophysiol. 2010 Oct;3 (5):481–8. doi: 10.1161/CIRCEP.110.954149. [DOI] [PubMed] [Google Scholar]
- 8.Packer Douglas L, Kowal Robert C, Wheelan Kevin R, Irwin James M, Champagne Jean, Guerra Peter G, Dubuc Marc, Reddy Vivek, Nelson Linda, Holcomb Richard G, Lehmann John W, Ruskin Jeremy N. Cryoballoon ablation of pulmonary veins for paroxysmal atrial fibrillation: first results of the North American Arctic Front (STOP AF) pivotal trial. J. Am. Coll. Cardiol. 2013 Apr 23;61 (16):1713–23. doi: 10.1016/j.jacc.2012.11.064. [DOI] [PubMed] [Google Scholar]
- 9.Neven Kars, Metzner Andreas, Schmidt Boris, Ouyang Feifan, Kuck Karl-Heinz. Two-year clinical follow-up after pulmonary vein isolation using high-intensity focused ultrasound (HIFU) and an esophageal temperature-guided safety algorithm. Heart Rhythm. 2012 Mar;9 (3):407–13. doi: 10.1016/j.hrthm.2011.09.072. [DOI] [PubMed] [Google Scholar]
- 10.Snyder KK, Baust JG, Baust JM, Gage AA. Mechanisms of cryoablation. In: Bredikis A, Wilber D, ed. Cryoablation of Cardiac Arrhythmias. Philadelphia, PA: Saunders. 2011;0:13–21. [Google Scholar]
- 11.Gage AA, Baust JM, Baust JG. Principles of cryosurgery. In: Rukstalis D, ed. Handbook of Urological Cryoablation. Abingdon, United Kingdom. Informa Healthcare. 2007;0:1–18. [Google Scholar]
- 12.Lustgarten DL. History of cardiac cryosurgery and cryoablation. In: Bredikis A, Wilber D, ed. Cryoablation of Cardiac Arrhythmias. Philadelphia, PA: Saunders. 2011;0:3–12. [Google Scholar]
- 13.Piccini Jonathan P, Daubert James P. Cryoablation of atrial fibrillation. J Interv Card Electrophysiol. 2011 Dec;32 (3):233–42. doi: 10.1007/s10840-011-9603-z. [DOI] [PubMed] [Google Scholar]
- 14. A Bredikis, D Wilber. Factors that determine cryolesion formation and cryolesion characteristics. In: Bredikis A, Wilber D, ed. Cryoablation of Cardiac Arrhythmias. Philadelphia,PA:Saunders; 2011;0:22–39. [Google Scholar]
- 15.Khairy Paul, Chauvet Patrick, Lehmann John, Lambert Jean, Macle Laurent, Tanguay Jean-François, Sirois Martin G, Santoianni Domenic, Dubuc Marc. Lower incidence of thrombus formation with cryoenergy versus radiofrequency catheter ablation. Circulation. 2003 Apr 22;107 (15):2045–50. doi: 10.1161/01.CIR.0000058706.82623.A1. [DOI] [PubMed] [Google Scholar]
- 16.Ghosh Justin, Martin Andrew, Keech Anthony C, Chan Kim H, Gomes Sean, Singarayar Suresh, McGuire Mark A. Balloon warming time is the strongest predictor of late pulmonary vein electrical reconnection following cryoballoon ablation for atrial fibrillation. Heart Rhythm. 2013 Sep;10 (9):1311–7. doi: 10.1016/j.hrthm.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 17.Ciconte Giuseppe, Chierchia Gian-Battista, DE Asmundis Carlo, Sieira Juan, Conte Giulio, Juliá Justo, DI Giovanni Giacomo, Wauters Kristel, Baltogiannis Giannis, Saitoh Yukio, Mugnai Giacomo, Catanzariti Domenico, Tondo Claudio, Brugada Pedro. Spontaneous and adenosine-induced pulmonary vein reconnection after cryoballoon ablation with the second-generation device. J. Cardiovasc. Electrophysiol. 2014 Aug;25 (8):845–51. doi: 10.1111/jce.12421. [DOI] [PubMed] [Google Scholar]
- 18.Tse Hung-Fat, Reek Sven, Timmermans Carl, Lee Kathy Lai-Fun, Geller J Christoph, Rodriguez Luz-Maria, Ghaye Benoit, Ayers Gregory M, Crijns Harry J G M, Klein Helmut U, Lau Chu-Pak. Pulmonary vein isolation using transvenous catheter cryoablation for treatment of atrial fibrillation without risk of pulmonary vein stenosis. J. Am. Coll. Cardiol. 2003 Aug 20;42 (4):752–8. doi: 10.1016/s0735-1097(03)00788-5. [DOI] [PubMed] [Google Scholar]
- 19.Hoyt Robert H, Wood Mark, Daoud Emile, Feld Gregory, Sehra Ruchir, Pelkey William, Kay G Neal, Calkins Hugh. Transvenous catheter cryoablation for treatment of atrial fibrillation: results of a feasibility study. Pacing Clin Electrophysiol. 2005 Jan;28 Suppl 1 ():S78–82. doi: 10.1111/j.1540-8159.2005.00060.x. [DOI] [PubMed] [Google Scholar]
- 20.Kenigsberg David N, Wood Mark A, Alaeddini Jamshid, Ellenbogen Kenneth A. Cryoablation inside the pulmonary vein after failure of radiofrequency antral isolation. Heart Rhythm. 2007 Aug;4 (8):992–6. doi: 10.1016/j.hrthm.2007.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Ahmed Humera, Neuzil Petr, d'Avila Andre, Cha Yong-Mei, Laragy Margaret, Mares Karel, Brugge William R, Forcione David G, Ruskin Jeremy N, Packer Douglas L, Reddy Vivek Y. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm. 2009 Jul;6 (7):962–9. doi: 10.1016/j.hrthm.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 22.Kuniss Malte, Vogtmann Thomas, Ventura Rodolfo, Willems Stephan, Vogt Jürgen, Grönefeld Gerian, Hohnloser Stefan, Zrenner Bernhard, Erdogan Ali, Klein Gunnar, Lemke Bernd, Neuzner Jörg, Neumann Thomas, Hamm Christian W, Pitschner Heinz-Friedrich. Prospective randomized comparison of durability of bidirectional conduction block in the cavotricuspid isthmus in patients after ablation of common atrial flutter using cryothermy and radiofrequency energy: the CRYOTIP study. Heart Rhythm. 2009 Dec;6 (12):1699–705. doi: 10.1016/j.hrthm.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Feld Gregory K, Yao Biguang. Evaluation of the safety and effectiveness of the CryoMedix cryoablation catheter system for the treatment of atrial flutter and fibrillation. J Interv Card Electrophysiol. 2014 Jan;39 (1):37–44. doi: 10.1007/s10840-013-9847-x. [DOI] [PubMed] [Google Scholar]
- 24.Skanes Allan C, Jensen Steen M, Papp Robert, Li Juliana, Yee Raymond, Krahn Andrew D, Klein George J. Isolation of pulmonary veins using a transvenous curvilinear cryoablation catheter: feasibility, initial experience, and analysis of recurrences. J. Cardiovasc. Electrophysiol. 2005 Dec;16 (12):1304–8. doi: 10.1111/j.1540-8167.2005.00287.x. [DOI] [PubMed] [Google Scholar]
- 25.Avitall Boaz, Urboniene Dalia, Rozmus Grzegorz, Lafontaine Dan, Helms Ray, Urbonas Arvydas. New cryotechnology for electrical isolation of the pulmonary veins. J. Cardiovasc. Electrophysiol. 2003 Mar;14 (3):281–6. doi: 10.1046/j.1540-8167.2003.02357.x. [DOI] [PubMed] [Google Scholar]
- 26.Sarabanda Alvaro V, Bunch T Jared, Johnson Susan B, Mahapatra Srijoy, Milton Mark A, Leite Luiz R, Bruce G Keith, Packer Douglas L. Efficacy and safety of circumferential pulmonary vein isolation using a novel cryothermal balloon ablation system. J. Am. Coll. Cardiol. 2005 Nov 15;46 (10):1902–12. doi: 10.1016/j.jacc.2005.07.046. [DOI] [PubMed] [Google Scholar]
- 27.Doshi SK, Laragy M, Pitchner H, Pitchner HF, Irwin J, Cole C, Neuzil P, Kuniss M, Ruskin JN, Reddy VY. The additive efficacy of a novel balloon cryoablation catheter to standard cryoablation for PV isolation in patients with symptomatic atrial fibrillation. . Heart Rhythm. 2005;2:0–0. [Google Scholar]
- 28.Malmborg Helena, Lönnerholm Stefan, Blomström Per, Blomström-Lundqvist Carina. Ablation of atrial fibrillation with cryoballoon or duty-cycled radiofrequency pulmonary vein ablation catheter: a randomized controlled study comparing the clinical outcome and safety; the AF-COR study. Europace. 2013 Nov;15 (11):1567–73. doi: 10.1093/europace/eut104. [DOI] [PubMed] [Google Scholar]
- 29.Neumann Thomas, Vogt Jürgen, Schumacher Burghard, Dorszewski Anja, Kuniss Malte, Neuser Hans, Kurzidim Klaus, Berkowitsch Alexander, Koller Marcus, Heintze Johannes, Scholz Ursula, Wetzel Ulrike, Schneider Michael A E, Horstkotte Dieter, Hamm Christian W, Pitschner Heinz-Friedrich. Circumferential pulmonary vein isolation with the cryoballoon technique results from a prospective 3-center study. J. Am. Coll. Cardiol. 2008 Jul 22;52 (4):273–8. doi: 10.1016/j.jacc.2008.04.021. [DOI] [PubMed] [Google Scholar]
- 30.Klein Gunnar, Oswald Hanno, Gardiwal Ajmal, Lüsebrink Ulrich, Lissel Christoph, Yu Hong, Drexler Helmut. Efficacy of pulmonary vein isolation by cryoballoon ablation in patients with paroxysmal atrial fibrillation. Heart Rhythm. 2008 Jun;5 (6):802–6. doi: 10.1016/j.hrthm.2008.02.014. [DOI] [PubMed] [Google Scholar]
- 31.Van Belle Yves, Janse Petter, Theuns Dominic, Szili-Torok Tamas, Jordaens Luc. One year follow-up after cryoballoon isolation of the pulmonary veins in patients with paroxysmal atrial fibrillation. Europace. 2008 Nov;10 (11):1271–6. doi: 10.1093/europace/eun218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chun Kyoung-Ryul Julian, Schmidt Boris, Metzner Andreas, Tilz Roland, Zerm Thomas, Köster Ilka, Fürnkranz Alexander, Koektuerk Buelent, Konstantinidou Melanie, Antz Matthias, Ouyang Feifan, Kuck Karl Heinz. The 'single big cryoballoon' technique for acute pulmonary vein isolation in patients with paroxysmal atrial fibrillation: a prospective observational single centre study. Eur. Heart J. 2009 Mar;30 (6):699–709. doi: 10.1093/eurheartj/ehn570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Defaye Pascal, Kane Adama, Chaib Ali, Jacon Peggy. Efficacy and safety of pulmonary veins isolation by cryoablation for the treatment of paroxysmal and persistent atrial fibrillation. Europace. 2011 Jun;13 (6):789–95. doi: 10.1093/europace/eur036. [DOI] [PubMed] [Google Scholar]
- 34.Vogt Jürgen, Heintze Johannes, Gutleben Klaus J, Muntean Bogdan, Horstkotte Dieter, Nölker Georg. Long-term outcomes after cryoballoon pulmonary vein isolation: results from a prospective study in 605 patients. J. Am. Coll. Cardiol. 2013 Apr 23;61 (16):1707–12. doi: 10.1016/j.jacc.2012.09.033. [DOI] [PubMed] [Google Scholar]
- 35.Ferrero-de Loma-Osorio Angel, Izquierdo-de Francisco Maite, Martínez-Brotons Angel, Sánchez-Gómez Juan M, Mascarell-Gregori Beatriz, Ruiz-Ros Vicente, Cuenca-Romero Isabel, García-Civera Roberto, Chorro-Gascó Francisco J, Ruiz-Granell Ricardo. Medium-term results of cryoballoon ablation of the pulmonary veins in patients with paroxysmal and persistent atrial fibrillation. First experience of a Spanish center. J Interv Card Electrophysiol. 2013 Aug;37 (2):189–96. doi: 10.1007/s10840-013-9797-3. [DOI] [PubMed] [Google Scholar]
- 36.Aytemir Kudret, Oto Ali, Canpolat Uğur, Sunman Hamza, Yorgun Hikmet, Şahiner Levent, Kaya Ergün Barış. Immediate and medium-term outcomes of cryoballoon-based pulmonary vein isolation in patients with paroxysmal and persistent atrial fibrillation: single-centre experience. J Interv Card Electrophysiol. 2013 Dec;38 (3):187–95. doi: 10.1007/s10840-013-9834-2. [DOI] [PubMed] [Google Scholar]
- 37.Rao Jayakeerthi Yoganarasimha, Chierchia Gian-Battista, de Asmundis Carlo, Casado-Arroyo Ruben, Overeinder Ingrid, Sarkozy Andrea, Paparella Gaetano, Capulzini Lucio, Sorgente Antonio, Rodriguez-Manero Moises, Ricciardi Danilo, Namdar Mehdi, Brugada Pedro. Cryoballoon ablation as index procedure for paroxysmal atrial fibrillation: long-term results from a single center early experience. J Cardiovasc Med (Hagerstown) 2014 Mar;15 (3):194–8. doi: 10.2459/JCM.0b013e3283623838. [DOI] [PubMed] [Google Scholar]
- 38.Andrade Jason G, Khairy Paul, Guerra Peter G, Deyell Marc W, Rivard Lena, Macle Laurent, Thibault Bernard, Talajic Mario, Roy Denis, Dubuc Marc. Efficacy and safety of cryoballoon ablation for atrial fibrillation: a systematic review of published studies. Heart Rhythm. 2011 Sep;8 (9):1444–51. doi: 10.1016/j.hrthm.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 39.Yorgun Hikmet, Aytemir Kudret, Canpolat Uğur, Şahiner Levent, Kaya Ergun Barış, Oto Ali. Additional benefit of cryoballoon-based atrial fibrillation ablation beyond pulmonary vein isolation: modification of ganglionated plexi. Europace. 2014 May;16 (5):645–51. doi: 10.1093/europace/eut240. [DOI] [PubMed] [Google Scholar]
- 40.Fürnkranz Alexander, Chun K R Julian, Nuyens Dieter, Metzner Andreas, Köster Ilka, Schmidt Boris, Ouyang Feifan, Kuck Karl-Heinz. Characterization of conduction recovery after pulmonary vein isolation using the "single big cryoballoon" technique. Heart Rhythm. 2010;7 (2):184–90. doi: 10.1016/j.hrthm.2009.10.038. [DOI] [PubMed] [Google Scholar]
- 41.Takami Mitsuru, Misiri Juna, Lehmann H Immo, Parker Kay D, Johnson Susan B, Sarmiento Ray I, Packer Douglas L. Spatial and time-course thermodynamics during pulmonary vein isolation using the second-generation cryoballoon in a canine in vivo model. Circ Arrhythm Electrophysiol. 2015 Feb;8 (1):186–92. doi: 10.1161/CIRCEP.114.002137. [DOI] [PubMed] [Google Scholar]
- 42.Knecht Sven, Kühne Michael, Osswald Stefan, Sticherling Christian. Quantitative assessment of a second-generation cryoballoon ablation catheter with new cooling technology-a perspective on potential implications on outcome. J Interv Card Electrophysiol. 2014 Jun;40 (1):17–21. doi: 10.1007/s10840-014-9883-1. [DOI] [PubMed] [Google Scholar]
- 43.Coulombe Nicolas, Paulin Jaime, Su Wilber. Improved in vivo performance of second-generation cryoballoon for pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2013 Aug;24 (8):919–25. doi: 10.1111/jce.12157. [DOI] [PubMed] [Google Scholar]
- 44.Andrade Jason G, Dubuc Marc, Guerra Peter G, Landry Evelyn, Coulombe Nicolas, Leduc Hugues, Rivard Léna, Macle Laurent, Thibault Bernard, Talajic Mario, Roy Denis, Khairy Paul. Pulmonary vein isolation using a second-generation cryoballoon catheter: a randomized comparison of ablation duration and method of deflation. J. Cardiovasc. Electrophysiol. 2013 Jun;24 (6):692–8. doi: 10.1111/jce.12114. [DOI] [PubMed] [Google Scholar]
- 45.Kenigsberg David N, Martin Natalia, Lim Hae W, Kowalski Marcin, Ellenbogen Kenneth A. Quantification of the cryoablation zone demarcated by pre- and postprocedural electroanatomic mapping in patients with atrial fibrillation using the 28-mm second-generation cryoballoon. Heart Rhythm. 2015 Feb;12 (2):283–90. doi: 10.1016/j.hrthm.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 46.Reddy Vivek Y, Sediva Lucie, Petru Jan, Skoda Jan, Chovanec Milan, Chitovova Zita, Di Stefano Paola, Rubin Ethel, Dukkipati Srinivas, Neuzil Petr. Durability of Pulmonary Vein Isolation with Cryoballoon Ablation: Results from the Sustained PV Isolation with Arctic Front Advance (SUPIR) Study. J. Cardiovasc. Electrophysiol. 2015 May;26 (5):493–500. doi: 10.1111/jce.12626. [DOI] [PubMed] [Google Scholar]
- 47.Martins Raphaël P, Hamon David, Césari Olivier, Behaghel Albin, Behar Nathalie, Sellal Jean-Marc, Daubert Jean-Claude, Mabo Philippe, Pavin Dominique. Safety and efficacy of a second-generation cryoballoon in the ablation of paroxysmal atrial fibrillation. Heart Rhythm. 2014 Mar;11 (3):386–93. doi: 10.1016/j.hrthm.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 48.Fürnkranz Alexander, Bordignon Stefano, Schmidt Boris, Gunawardene Melanie, Schulte-Hahn Britta, Urban Verena, Bode Frank, Nowak Bernd, Chun Julian K R. Improved procedural efficacy of pulmonary vein isolation using the novel second-generation cryoballoon. J. Cardiovasc. Electrophysiol. 2013 May;24 (5):492–7. doi: 10.1111/jce.12082. [DOI] [PubMed] [Google Scholar]
- 49.Aryana Arash, Morkoch Shemsa, Bailey Sean, Lim Hae W, Sara Rahmani, d'Avila André, O'Neill P Gearoid. Acute procedural and cryoballoon characteristics from cryoablation of atrial fibrillation using the first- and second-generation cryoballoon: a retrospective comparative study with follow-up outcomes. J Interv Card Electrophysiol. 2014 Nov;41 (2):177–86. doi: 10.1007/s10840-014-9942-7. [DOI] [PubMed] [Google Scholar]
- 50.Straube Florian, Dorwarth Uwe, Schmidt Martin, Wankerl Michael, Ebersberger Ulrich, Hoffmann Ellen. Comparison of the first and second cryoballoon: high-volume single-center safety and efficacy analysis. Circ Arrhythm Electrophysiol. 2014 Apr;7 (2):293–9. doi: 10.1161/CIRCEP.113.000899. [DOI] [PubMed] [Google Scholar]
- 51.Fürnkranz Alexander, Bordignon Stefano, Dugo Daniela, Perotta Laura, Gunawardene Melanie, Schulte-Hahn Britta, Nowak Bernd, Schmidt Boris, Chun Julian K R. Improved 1-year clinical success rate of pulmonary vein isolation with the second-generation cryoballoon in patients with paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2014 Aug;25 (8):840–4. doi: 10.1111/jce.12417. [DOI] [PubMed] [Google Scholar]
- 52.Di Giovanni Giacomo, Wauters Kristel, Chierchia Gian-Battista, Sieira Juan, Levinstein Moises, Conte Giulio, de Asmundis Carlo, Baltogiannis Giannis, Saitoh Yukio, Ciconte Giuseppe, Julia Justo, Mugnai Giacomo, Irfan Ghazala, Brugada Pedro. One-year follow-up after single procedure Cryoballoon ablation: a comparison between the first and second generation balloon. J. Cardiovasc. Electrophysiol. 2014 Aug;25 (8):834–9. doi: 10.1111/jce.12409. [DOI] [PubMed] [Google Scholar]
- 53.Liu Jun, Kaufmann Jan, Kriatselis Charalampos, Fleck Eckart, Gerds-Li Jin Hong. Second generation of cryoballoons can improve efficiency of cryoablation for atrial fibrillation. Pacing Clin Electrophysiol. 2015 Jan;38 (1):129–35. doi: 10.1111/pace.12538. [DOI] [PubMed] [Google Scholar]
- 54.Bordignon Stefano, Fürnkranz Alexander, Dugo Daniela, Perrotta Laura, Gunawardene Melanie, Bode Frank, Klemt Anne, Nowak Bernd, Schulte-Hahn Britta, Schmidt Boris, Chun K R Julian. Improved lesion formation using the novel 28 mm cryoballoon in atrial fibrillation ablation: analysis of biomarker release. Europace. 2014 Jul;16 (7):987–93. doi: 10.1093/europace/eut400. [DOI] [PubMed] [Google Scholar]
- 55.Chierchia Gian-Battista, Di Giovanni Giacomo, Ciconte Giuseppe, de Asmundis Carlo, Conte Giulio, Sieira-Moret Juan, Rodriguez-Mañero Moises, Casado Ruben, Baltogiannis Giannis, Namdar Mehdi, Saitoh Yukio, Paparella Gaetano, Mugnai Giacomo, Brugada Pedro. Second-generation cryoballoon ablation for paroxysmal atrial fibrillation: 1-year follow-up. Europace. 2014 May;16 (5):639–44. doi: 10.1093/europace/eut417. [DOI] [PubMed] [Google Scholar]
- 56.Metzner Andreas, Reissmann Bruno, Rausch Peter, Mathew Shibu, Wohlmuth Peter, Tilz Roland, Rillig Andreas, Lemes Christine, Deiss Sebastian, Heeger Christian, Kamioka Masashi, Lin Tina, Ouyang Feifan, Kuck Karl-Heinz, Wissner Erik. One-year clinical outcome after pulmonary vein isolation using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol. 2014 Apr;7 (2):288–92. doi: 10.1161/CIRCEP.114.001473. [DOI] [PubMed] [Google Scholar]
- 57.Ciconte Giuseppe, de Asmundis Carlo, Sieira Juan, Conte Giulio, Di Giovanni Giacomo, Mugnai Giacomo, Saitoh Yukio, Baltogiannis Giannis, Irfan Ghazala, Coutiño-Moreno Hugo Enrique, Hunuk Burak, Velagić Vedran, Brugada Pedro, Chierchia Gian-Battista. Single 3-minute freeze for second-generation cryoballoon ablation: one-year follow-up after pulmonary vein isolation. Heart Rhythm. 2015 Apr;12 (4):673–80. doi: 10.1016/j.hrthm.2014.12.026. [DOI] [PubMed] [Google Scholar]
- 58.Aryana Arash, Singh Sheldon M, Kowalski Marcin, Pujara Deep K, Cohen Andrew I, Singh Steve K, Aleong Ryan G, Banker Rajesh S, Fuenzalida Charles E, Prager Nelson A, Bowers Mark R, D'Avila André, O'Neill Padraig Gearoid. Acute and Long-Term Outcomes of Catheter Ablation of Atrial Fibrillation Using the Second-Generation Cryoballoon versus Open-Irrigated Radiofrequency: A Multicenter Experience. J. Cardiovasc. Electrophysiol. 2015 Aug;26 (8):832–9. doi: 10.1111/jce.12695. [DOI] [PubMed] [Google Scholar]
- 59.Chierchia Gian-Battista, de Asmundis Carlo, Namdar Mehdi, Westra Sjoerd, Kuniss Malte, Sarkozy Andrea, Bayrak Fatih, Ricciardi Danilo, Casado-Arroyo Ruben, Rodriguez Manero Moises, Rao Jayakeerthi Y, Smeets Joep, Brugada Pedro. Pulmonary vein isolation during cryoballoon ablation using the novel Achieve inner lumen mapping catheter: a feasibility study. Europace. 2012 Jul;14 (7):962–7. doi: 10.1093/europace/eus041. [DOI] [PubMed] [Google Scholar]
- 60.Kühne Michael, Knecht Sven, Altmann David, Ammann Peter, Schaer Beat, Osswald Stefan, Sticherling Christian. Validation of a novel spiral mapping catheter for real-time recordings from the pulmonary veins during cryoballoon ablation of atrial fibrillation. Heart Rhythm. 2013 Feb;10 (2):241–6. doi: 10.1016/j.hrthm.2012.10.009. [DOI] [PubMed] [Google Scholar]
- 61.Boveda Serge, Providência Rui, Albenque Jean-Paul, Combes Nicolas, Combes Stéphane, Hireche Hassiba, Casteigt Benjamin, Bouzeman Abdeslam, Jourda François, Narayanan Kumar, Marijon Eloi. Real-time assessment of pulmonary vein disconnection during cryoablation of atrial fibrillation: can it be 'achieved' in almost all cases? Europace. 2014 Jun;16 (6):826–33. doi: 10.1093/europace/eut366. [DOI] [PubMed] [Google Scholar]
- 62.Sorgente Antonio, Chierchia Gian Battista, de Asmundis Carlo, Sarkozy Andrea, Namdar Mehdi, Capulzini Lucio, Yazaki Yoshinao, Müller-Burri Stephan-Andreas, Bayrak Fatih, Brugada Pedro. Pulmonary vein ostium shape and orientation as possible predictors of occlusion in patients with drug-refractory paroxysmal atrial fibrillation undergoing cryoballoon ablation. Europace. 2011 Feb;13 (2):205–12. doi: 10.1093/europace/euq388. [DOI] [PubMed] [Google Scholar]
- 63.Kubala Maciej, Hermida Jean-Sylvain, Nadji Georges, Quenum Serge, Traulle Sarah, Jarry Geneviève. Normal pulmonary veins anatomy is associated with better AF-free survival after cryoablation as compared to atypical anatomy with common left pulmonary vein. Pacing Clin Electrophysiol. 2011 Jul;34 (7):837–43. doi: 10.1111/j.1540-8159.2011.03070.x. [DOI] [PubMed] [Google Scholar]
- 64.Ghosh Justin, Martin Andrew, Keech Anthony C, Chan Kim H, Gomes Sean, Singarayar Suresh, McGuire Mark A. Balloon warming time is the strongest predictor of late pulmonary vein electrical reconnection following cryoballoon ablation for atrial fibrillation. Heart Rhythm. 2013 Sep;10 (9):1311–7. doi: 10.1016/j.hrthm.2013.06.014. [DOI] [PubMed] [Google Scholar]
- 65.Aryana A, Lim HW, Kowalski M, Hokanson RB, Parikh V, Torbey E, Bowers MR, O’Neill PG. With catheter ablation using the second-generation cryoballoon only thaw time and freeze area-under-the-curve were predictive of long-term freedom from atrial fibrillation. Heart Rhythm. 2015;12:0–0. [Google Scholar]
- 66.Fürnkranz Alexander, Köster Ilka, Chun K R Julian, Metzner Andreas, Mathew Shibu, Konstantinidou Melanie, Ouyang Feifan, Kuck Karl Heinz. Cryoballoon temperature predicts acute pulmonary vein isolation. Heart Rhythm. 2011 Jun;8 (6):821–5. doi: 10.1016/j.hrthm.2011.01.044. [DOI] [PubMed] [Google Scholar]
- 67.Andrade Jason G, Khairy Paul, Macle Laurent, Packer Doug L, Lehmann John W, Holcomb Richard G, Ruskin Jeremy N, Dubuc Marc. Incidence and significance of early recurrences of atrial fibrillation after cryoballoon ablation: insights from the multicenter Sustained Treatment of Paroxysmal Atrial Fibrillation (STOP AF) Trial. Circ Arrhythm Electrophysiol. 2014 Feb;7 (1):69–75. doi: 10.1161/CIRCEP.113.000586. [DOI] [PubMed] [Google Scholar]
- 68.Linhart Markus, Bellmann Barbara, Mittmann-Braun Erica, Schrickel Jan W, Bitzen Alexander, Andrié René, Yang Alexander, Nickenig Georg, Lickfett Lars, Lewalter Thorsten. Comparison of cryoballoon and radiofrequency ablation of pulmonary veins in 40 patients with paroxysmal atrial fibrillation: a case-control study. J. Cardiovasc. Electrophysiol. 2009 Dec;20 (12):1343–8. doi: 10.1111/j.1540-8167.2009.01560.x. [DOI] [PubMed] [Google Scholar]
- 69.Kojodjojo Pipin, O'Neill Mark D, Lim Phang Boon, Malcolm-Lawes Louisa, Whinnett Zachary I, Salukhe Tushar V, Linton Nicholas W, Lefroy David, Mason Anthony, Wright Ian, Peters Nicholas S, Kanagaratnam Prapa, Davies D Wyn. Pulmonary venous isolation by antral ablation with a large cryoballoon for treatment of paroxysmal and persistent atrial fibrillation: medium-term outcomes and non-randomised comparison with pulmonary venous isolation by radiofrequency ablation. Heart. 2010 Sep;96 (17):1379–84. doi: 10.1136/hrt.2009.192419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Tayebjee Muzahir H, Hunter Ross J, Baker Victoria, Creta Antonio, Duncan Edward, Sporton Simon, Earley Mark J, Schilling Richard J. Pulmonary vein isolation with radiofrequency ablation followed by cryotherapy: a novel strategy to improve clinical outcomes following catheter ablation of paroxysmal atrial fibrillation. Europace. 2011 Sep;13 (9):1250–5. doi: 10.1093/europace/eur140. [DOI] [PubMed] [Google Scholar]
- 71.Kühne Michael, Suter Yves, Altmann David, Ammann Peter, Schaer Beat, Osswald Stefan, Sticherling Christian. Cryoballoon versus radiofrequency catheter ablation of paroxysmal atrial fibrillation: biomarkers of myocardial injury, recurrence rates, and pulmonary vein reconnection patterns. Heart Rhythm. 2010 Dec;7 (12):1770–6. doi: 10.1016/j.hrthm.2010.08.028. [DOI] [PubMed] [Google Scholar]
- 72.Sorgente Antonio, Chierchia Gian Battista, Capulzini Lucio, Yazaki Yoshinao, Muller-Burri Andreas, Bayrak Fatih, Sarkozy Andrea, de Asmundis Carlo, Paparella Gaetano, Brugada Brugada. Atrial fibrillation ablation: a single center comparison between remote magnetic navigation, cryoballoon and conventional manual pulmonary vein isolation. Indian Pacing Electrophysiol J. 2010;10 (11):486–95. [PMC free article] [PubMed] [Google Scholar]
- 73.Herrera Siklódy Claudia, Arentz Thomas, Minners Jan, Jesel Laurence, Stratz Christian, Valina Christian M, Weber Reinhold, Kalusche Dietrich, Toti Florence, Morel Olivier, Trenk Dietmar. Cellular damage, platelet activation, and inflammatory response after pulmonary vein isolation: a randomized study comparing radiofrequency ablation with cryoablation. Heart Rhythm. 2012 Feb;9 (2):189–96. doi: 10.1016/j.hrthm.2011.09.017. [DOI] [PubMed] [Google Scholar]
- 74.Schmidt Martin, Dorwarth Uwe, Andresen Dietrich, Brachmann Johannes, Kuck Karl-Heinz, Kuniss Malte, Lewalter Thorsten, Spitzer Stefan, Willems Stephan, Senges Jochen, Jünger Claus, Hoffmann Ellen. Cryoballoon versus RF ablation in paroxysmal atrial fibrillation: results from the German Ablation Registry. J. Cardiovasc. Electrophysiol. 2014 Jan;25 (1):1–7. doi: 10.1111/jce.12267. [DOI] [PubMed] [Google Scholar]
- 75.Mugnai Giacomo, Chierchia Gian-Battista, de Asmundis Carlo, Sieira-Moret Juan, Conte Giulio, Capulzini Lucio, Wauters Kristel, Rodriguez-Mañero Moises, Di Giovanni Giacomo, Baltogiannis Giannis, Ciconte Giuseppe, Saitoh Yukio, Juliá Justo, Brugada Pedro. Comparison of pulmonary vein isolation using cryoballoon versus conventional radiofrequency for paroxysmal atrial fibrillation. Am. J. Cardiol. 2014 May 1;113 (9):1509–13. doi: 10.1016/j.amjcard.2014.01.425. [DOI] [PubMed] [Google Scholar]
- 76.Juliá Justo, Chierchia Gian-Battista, de Asmundis Carlo, Mugnai Giacomo, Sieira Juan, Ciconte Giuseppe, Di Giovanni Giacomo, Conte Giulio, Baltogiannis Giannis, Saitoh Yukio, Wauters Kristel, Irfan Ghazala, Brugada Pedro. Regular atrial tachycardias following pulmonary vein isolation for paroxysmal atrial fibrillation: a retrospective comparison between the cryoballoon and conventional focal tip radiofrequency techniques. J Interv Card Electrophysiol. 2015 Mar;42 (2):161–9. doi: 10.1007/s10840-014-9961-4. [DOI] [PubMed] [Google Scholar]
- 77.Xu Junxia, Huang Yingqun, Cai Hongbin, Qi Yue, Jia Nan, Shen Weifeng, Lin Jinxiu, Peng Feng, Niu Wenquan. Is cryoballoon ablation preferable to radiofrequency ablation for treatment of atrial fibrillation by pulmonary vein isolation? A meta-analysis. PLoS ONE. 2014;9 (2) doi: 10.1371/journal.pone.0090323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chinitz Jason S, Kulina Robert A, Gangireddy Sandeep R, Miller Marc A, Koruth Jacob S, Dukkipati Srinivas R, Halperin Jonathan L, Reddy Vivek Y, d'Avila Andre. Objective quality assessment of atrial fibrillation ablation: a novel scoring system. Heart Rhythm. 2013 Jul;10 (7):1074–9. doi: 10.1016/j.hrthm.2013.02.026. [DOI] [PubMed] [Google Scholar]
- 79.Jourda François, Providencia Rui, Marijon Eloi, Bouzeman Abdeslam, Hireche Hassiba, Khoueiry Ziad, Cardin Christelle, Combes Nicolas, Combes Stéphane, Boveda Serge, Albenque Jean-Paul. Contact-force guided radiofrequency vs. second-generation balloon cryotherapy for pulmonary vein isolation in patients with paroxysmal atrial fibrillation-a prospective evaluation. Europace. 2015 Feb;17 (2):225–31. doi: 10.1093/europace/euu215. [DOI] [PubMed] [Google Scholar]
- 80.Torbey E, Parikh V, Agarwal V, Bekheit S, Kowalski M. Treatment of long persistent atrial fibrillation by single cryoballoon ablation of the pulmonary veins, LA roof line and ablation of left lateral ridge from within the appendage. Heart Rhythm. 2014;11:0–0. [Google Scholar]
- 81.Frazier DW, Barrett KG. Linear lesions with Medtronic cryoballoon – posterior left atrial wall isolation. AF symposium. 2015;93:495–499. [Google Scholar]
- 82.Torbey E, Aryana A, Parikh V, Agarwal V, Bekheit S, O’Neill PG, Kowalski M. Treatment of Long-standing persistent atrial fibrillation by single cryoballoon ablation of the pulmonary veins, left atrial roof and left lateral ridge lesions. Chest . 2015;0:0–0. [Google Scholar]
- 83.Kumar Narendra, Pison Laurent, La Meir Mark, Maessen Jos, Crijns Harry J. Hybrid approach to atrial fibrillation ablation using bipolar radiofrequency devices epicardially and cryoballoon endocardially. Interact Cardiovasc Thorac Surg. 2014 Oct;19 (4):590–4. doi: 10.1093/icvts/ivu189. [DOI] [PubMed] [Google Scholar]
- 84.Mittal S, Sperling JS, Arshad A, Musat D, Preminger M, Sichrovsky T, Steinberg JS. The efficacy of a novel hybrid ablation procedure in patients with refractory atrial fibrillation. ESC. 2014;93:0–499. [Google Scholar]
- 85.Bhat Tariq, Baydoun Hassan, Asti Deepak, Rijal Jharendra, Teli Sumaya, Tantray Mohmad, Bhat Hilal, Kowalski Marcin. Major complications of cryoballoon catheter ablation for atrial fibrillation and their management. Expert Rev Cardiovasc Ther. 2014 Sep;12 (9):1111–8. doi: 10.1586/14779072.2014.925802. [DOI] [PubMed] [Google Scholar]
- 86.Neumann Thomas, Kuniss Malte, Conradi Guido, Janin Sebastien, Berkowitsch Alexander, Wojcik Maciej, Rixe Johannes, Erkapic Damir, Zaltsberg Sergey, Rolf Andreas, Bachmann Georg, Dill Thorsten, Hamm Christian W, Pitschner Heinz-Friedrich. MEDAFI-Trial (Micro-embolization during ablation of atrial fibrillation): comparison of pulmonary vein isolation using cryoballoon technique vs. radiofrequency energy. Europace. 2011 Jan;13 (1):37–44. doi: 10.1093/europace/euq303. [DOI] [PubMed] [Google Scholar]
- 87.Chierchia Gian Battista, Capulzini Lucio, Droogmans Steven, Sorgente Antonio, Sarkozy Andrea, Müller-Burri Andreas, Paparella Gaetano, de Asmundis Carlo, Yazaki Yoshinao, Kerkhove Dirk, Van Camp Guy, Brugada Pedro. Pericardial effusion in atrial fibrillation ablation: a comparison between cryoballoon and radiofrequency pulmonary vein isolation. Europace. 2010 Mar;12 (3):337–41. doi: 10.1093/europace/eup422. [DOI] [PubMed] [Google Scholar]
- 88.Chan Ngai-Yin, Choy Chi-Chung, Lau Chun-Leung, Lo Ying-Keung, Chu Pui-Shan, Yuen Ho-Chuen, Mok Ngai-Shing, Tsui Ping-Tim, Lau Suet-Ting. Persistent iatrogenic atrial septal defect after pulmonary vein isolation by cryoballoon: an under-recognized complication. Europace. 2011 Oct;13 (10):1406–10. doi: 10.1093/europace/eur138. [DOI] [PubMed] [Google Scholar]
- 89.Cronin Edmond M, Collier Patrick, Wazni Oussama M, Griffin Brian P, Jaber Wael A, Saliba Walid I. Persistence of atrial septal defect after cryoballoon ablation of atrial fibrillation. J. Am. Coll. Cardiol. 2013 Oct 15;62 (16):1491–2. doi: 10.1016/j.jacc.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 90.Sieira Juan, Chierchia Gian-Battista, Di Giovanni Giacomo, Conte Giulio, De Asmundis Carlo, Sarkozy Andrea, Droogmans Steven, Baltogiannis Giannis, Saitoh Yukio, Ciconte Giusepe, Levinstein Moises, Brugada Pedro. One year incidence of iatrogenic atrial septal defect after cryoballoon ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2014 Jan;25 (1):11–5. doi: 10.1111/jce.12279. [DOI] [PubMed] [Google Scholar]
- 91.Sacher Frédéric, Monahan Kristi H, Thomas Stuart P, Davidson Neil, Adragao Pedro, Sanders Prashanthan, Hocini Mélèze, Takahashi Yoshihide, Rotter Martin, Rostock Thomas, Hsu Li-Fern, Clémenty Jacques, Haïssaguerre Michel, Ross David L, Packer Douglas L, Jaïs Pierre. Phrenic nerve injury after atrial fibrillation catheter ablation: characterization and outcome in a multicenter study. J. Am. Coll. Cardiol. 2006 Jun 20;47 (12):2498–503. doi: 10.1016/j.jacc.2006.02.050. [DOI] [PubMed] [Google Scholar]
- 92.Cappato Riccardo, Calkins Hugh, Chen Shih-Ann, Davies Wyn, Iesaka Yoshito, Kalman Jonathan, Kim You-Ho, Klein George, Packer Douglas, Skanes Allan. Worldwide survey on the methods, efficacy, and safety of catheter ablation for human atrial fibrillation. Circulation. 2005 Mar 8;111 (9):1100–5. doi: 10.1161/01.CIR.0000157153.30978.67. [DOI] [PubMed] [Google Scholar]
- 93.Andrade Jason G, Dubuc Marc, Ferreira Jose, Guerra Peter G, Landry Evelyn, Coulombe Nicolas, Rivard Lena, Macle Laurent, Thibault Bernard, Talajic Mario, Roy Denis, Khairy Paul. Histopathology of cryoballoon ablation-induced phrenic nerve injury. J. Cardiovasc. Electrophysiol. 2014 Feb;25 (2):187–94. doi: 10.1111/jce.12296. [DOI] [PubMed] [Google Scholar]
- 94.Casado-Arroyo Ruben, Chierchia Gian-Battista, Conte Giulio, Levinstein Moisés, Sieira Juan, Rodriguez-Mañero Moises, di Giovanni Giacomo, Baltogiannis Yannis, Wauters Kristel, de Asmundis Carlo, Sarkozy Andrea, Brugada Pedro. Phrenic nerve paralysis during cryoballoon ablation for atrial fibrillation: a comparison between the first- and second-generation balloon. Heart Rhythm. 2013 Sep;10 (9):1318–24. doi: 10.1016/j.hrthm.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 95.Andrade Jason G, Dubuc Marc, Guerra Peter G, Macle Laurent, Mondésert Blandine, Rivard Léna, Roy Denis, Talajic Mario, Thibault Bernard, Khairy Paul. The biophysics and biomechanics of cryoballoon ablation. Pacing Clin Electrophysiol. 2012 Sep;35 (9):1162–8. doi: 10.1111/j.1540-8159.2012.03436.x. [DOI] [PubMed] [Google Scholar]
- 96.Franceschi Frédéric, Dubuc Marc, Guerra Peter G, Delisle Stéphane, Romeo Philippe, Landry Evelyn, Koutbi Linda, Rivard Léna, Macle Laurent, Thibault Bernard, Talajic Mario, Roy Denis, Khairy Paul. Diaphragmatic electromyography during cryoballoon ablation: a novel concept in the prevention of phrenic nerve palsy. Heart Rhythm. 2011 Jun;8 (6):885–91. doi: 10.1016/j.hrthm.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 97.Pappone Carlo, Oral Hakan, Santinelli Vincenzo, Vicedomini Gabriele, Lang Christopher C, Manguso Francesco, Torracca Lucia, Benussi Stefano, Alfieri Ottavio, Hong Robert, Lau William, Hirata Kirk, Shikuma Neil, Hall Burr, Morady Fred. Atrio-esophageal fistula as a complication of percutaneous transcatheter ablation of atrial fibrillation. Circulation. 2004 Jun 8;109 (22):2724–6. doi: 10.1161/01.CIR.0000131866.44650.46. [DOI] [PubMed] [Google Scholar]
- 98.Borchert Bianca, Lawrenz Thorsten, Hansky Bert, Stellbrink Christoph. Lethal atrioesophageal fistula after pulmonary vein isolation using high-intensity focused ultrasound (HIFU). Heart Rhythm. 2008 Jan;5 (1):145–8. doi: 10.1016/j.hrthm.2007.08.023. [DOI] [PubMed] [Google Scholar]
- 99.Kawasaki Raymond, Gauri Andre, Elmouchi Darryl, Duggal Manoj, Bhan Adarsh. Atrioesophageal fistula complicating cryoballoon pulmonary vein isolation for paroxysmal atrial fibrillation. J. Cardiovasc. Electrophysiol. 2014 Jul;25 (7):787–92. doi: 10.1111/jce.12426. [DOI] [PubMed] [Google Scholar]
- 100.Lim Hae W, Cogert Gregory A, Cameron Craig S, Cheng Victor Y, Sandler David A. Atrioesophageal fistula during cryoballoon ablation for atrial fibrillation. J. Cardiovasc. Electrophysiol. 2014 Feb;25 (2):208–13. doi: 10.1111/jce.12313. [DOI] [PubMed] [Google Scholar]
- 101.Viladés Medel David, Marti-Almor Julio, Montiel Serrano Jose, Sionis Alessandro, Leta Petracca Rubén. Atrioesophageal fistula secondary to pulmonary vein cryo-ablation. Eur Heart J Cardiovasc Imaging. 2014 Jan;15 (1) doi: 10.1093/ehjci/jet143. [DOI] [PubMed] [Google Scholar]
- 102.Stöckigt Florian, Schrickel Jan W, Andrié René, Lickfett Lars. Atrioesophageal fistula after cryoballoon pulmonary vein isolation. J. Cardiovasc. Electrophysiol. 2012 Nov;23 (11):1254–7. doi: 10.1111/j.1540-8167.2012.02324.x. [DOI] [PubMed] [Google Scholar]
- 103.Grubina Rozalina, Cha Yong-Mei, Bell Malcolm R, Sinak Lawrence J, Asirvatham Samuel J. Pneumopericardium following radiofrequency ablation for atrial fibrillation: insights into the natural history of atrial esophageal fistula formation. J. Cardiovasc. Electrophysiol. 2010 Sep;21 (9):1046–9. doi: 10.1111/j.1540-8167.2010.01740.x. [DOI] [PubMed] [Google Scholar]
- 104.Fürnkranz Alexander, Chun K R Julian, Metzner Andreas, Nuyens Dieter, Schmidt Boris, Burchard Andre, Tilz Roland, Ouyang Feifan, Kuck Karl Heinz. Esophageal endoscopy results after pulmonary vein isolation using the single big cryoballoon technique. J. Cardiovasc. Electrophysiol. 2010 Aug 1;21 (8):869–74. doi: 10.1111/j.1540-8167.2010.01739.x. [DOI] [PubMed] [Google Scholar]
- 105.Ahmed Humera, Neuzil Petr, d'Avila Andre, Cha Yong-Mei, Laragy Margaret, Mares Karel, Brugge William R, Forcione David G, Ruskin Jeremy N, Packer Douglas L, Reddy Vivek Y. The esophageal effects of cryoenergy during cryoablation for atrial fibrillation. Heart Rhythm. 2009 Jul;6 (7):962–9. doi: 10.1016/j.hrthm.2009.03.051. [DOI] [PubMed] [Google Scholar]
- 106.Metzner Andreas, Burchard Andre, Wohlmuth Peter, Rausch Peter, Bardyszewski Alexander, Gienapp Christina, Tilz Roland Richard, Rillig Andreas, Mathew Shibu, Deiss Sebastian, Makimoto Hisaki, Ouyang Feifan, Kuck Karl-Heinz, Wissner Erik. Increased incidence of esophageal thermal lesions using the second-generation 28-mm cryoballoon. Circ Arrhythm Electrophysiol. 2013 Aug;6 (4):769–75. doi: 10.1161/CIRCEP.113.000228. [DOI] [PubMed] [Google Scholar]
- 107.Fürnkranz Alexander, Bordignon Stefano, Schmidt Boris, Böhmig Michael, Böhmer Marie-Christine, Bode Frank, Schulte-Hahn Britta, Nowak Bernd, Dignaß Axel U, Chun Julian K R. Luminal esophageal temperature predicts esophageal lesions after second-generation cryoballoon pulmonary vein isolation. Heart Rhythm. 2013 Jun;10 (6):789–93. doi: 10.1016/j.hrthm.2013.02.021. [DOI] [PubMed] [Google Scholar]
- 108.Zellerhoff Stephan, Ullerich Hansjörg, Lenze Frank, Meister Tobias, Wasmer Kristina, Mönnig Gerold, Köbe Julia, Milberg Peter, Bittner Alex, Domschke Wolfram, Breithardt Günter, Eckardt Lars. Damage to the esophagus after atrial fibrillation ablation: Just the tip of the iceberg? High prevalence of mediastinal changes diagnosed by endosonography. Circ Arrhythm Electrophysiol. 2010 Apr;3 (2):155–9. doi: 10.1161/CIRCEP.109.915918. [DOI] [PubMed] [Google Scholar]
- 109.Di Biase Luigi, Dodig Milan, Saliba Walid, Siu Alan, Santisi Janice, Poe Stacy, Sanaka Madhusudhan, Upchurch Bennie, Vargo John, Natale Andrea. Capsule endoscopy in examination of esophagus for lesions after radiofrequency catheter ablation: a potential tool to select patients with increased risk of complications. J. Cardiovasc. Electrophysiol. 2010 Aug 1;21 (8):839–44. doi: 10.1111/j.1540-8167.2010.01732.x. [DOI] [PubMed] [Google Scholar]
- 110.Schmidt Martin, Nölker Georg, Marschang Harald, Gutleben Klaus-Jürgen, Schibgilla Volker, Rittger Harald, Sinha Anil-Martin, Ritscher Guido, Mayer Dirk, Brachmann Johannes, Marrouche Nassir F. Incidence of oesophageal wall injury post-pulmonary vein antrum isolation for treatment of patients with atrial fibrillation. Europace. 2008 Feb;10 (2):205–9. doi: 10.1093/europace/eun001. [DOI] [PubMed] [Google Scholar]
- 111.Fürnkranz Alexander, Bordignon Stefano, Böhmig Michael, Konstantinou Athanasios, Dugo Daniela, Perrotta Laura, Klopffleisch Tom, Nowak Bernd, Dignaß Axel U, Schmidt Boris, Chun Julian K R. Reduced incidence of esophageal lesions by luminal esophageal temperature-guided second-generation cryoballoon ablation. Heart Rhythm. 2015 Feb;12 (2):268–74. doi: 10.1016/j.hrthm.2014.10.033. [DOI] [PubMed] [Google Scholar]
- 112.van Opstal J M, Timmermans C, Blaauw Y, Pison L. Bronchial erosion and hemoptysis after pulmonary vein isolation by cryoballoon ablation. Heart Rhythm. 2011 Sep;8 (9) doi: 10.1016/j.hrthm.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 113.Martí-Almor Julio, Jauregui-Abularach Miguel E, Benito Begoña, Vallès Ermengol, Bazan Victor, Sánchez-Font Albert, Vollmer Ivan, Altaba Carmen, Guijo Miguel A, Hervas Manel, Bruguera-Cortada Jordi. Pulmonary hemorrhage after cryoballoon ablation for pulmonary vein isolation in the treatment of atrial fibrillation. Chest. 2014 Jan;145 (1):156–7. doi: 10.1378/chest.13-0761. [DOI] [PubMed] [Google Scholar]
- 114.Kumar Narendra, Timmermans Carl, Das Marco, Dassen Willem, Philippens Suzanne, Maessen Jos, Crijns Harry J. Hemoptysis after cryoablation for atrial fibrillation: truth or just a myth? Chest. 2014 Nov;146 (5):e173–5. doi: 10.1378/chest.14-1600. [DOI] [PubMed] [Google Scholar]
- 115.J Vogt, J Heintze, G Noelker , KJ Gutleben , D Horstkotte. Long Term Efficacy and Side Effects of Antral Isolation of Pulmonary Veins With Cryoballoon Technique in a Large Patient Cohort With Atrial Fibrillation. Chest . 2009;120:0–0. [Google Scholar]
- 116.Bhagwandien Rohit, Van Belle Yves, de Groot Natasja, Jordaens Luc. Hemoptysis after pulmonary vein isolation with a cryoballoon: an analysis of the potential etiology. J. Cardiovasc. Electrophysiol. 2011 Sep;22 (9):1067–9. doi: 10.1111/j.1540-8167.2011.02031.x. [DOI] [PubMed] [Google Scholar]
- 117.Weig HJ, Weretka S, Parade U. Cryo-specific complications using the single big cryoballoon technique for pulmonary vein isolation in patients with paroxysmal atrial fibrillation. (abstract) Eur Heart J. 2010;0:556–0. [Google Scholar]
- 118.Malmborg Helena, Lönnerholm Stefan, Blomström-Lundqvist Carina. Acute and clinical effects of cryoballoon pulmonary vein isolation in patients with symptomatic paroxysmal and persistent atrial fibrillation. Europace. 2008 Nov;10 (11):1277–80. doi: 10.1093/europace/eun286. [DOI] [PubMed] [Google Scholar]