Abstract
Atrial fibrillation (AF) is the most common cardiac arrhythmia in athletes, especially in middle-aged athletes. Studies have demonstrated that athletes who engage in endurance sports such as runners, cyclists and skiers are more prone to AF than other athletes. The effects of exercise on the onset and progression of AF is complex. Triggers of AF in athletes may include atrial ectopy and sports supplements. Substrates for AF in athletes include atrial remodeling, fibrosis, and inflammation. Modulators of AF in athletes include autonomic activation, electrolyte abnormalities, and possibly, gastroesophageal reflux. Management of AF in athletes with rate-controlling agents and antiarrhythmic drugs remains a challenge and can be associated with impaired athletic performance. The value of catheter ablation is emerging and should be considered in suitable athletes with AF.
Keywords: Atrial Fibrillation, Athletes, Exercise, Pathophysiology, Evaluation, Management
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia in athletes, especially in middle-aged athletes.[1] Participation in regular physical exercise has been shown to be beneficial to cardiovascular health and overall well-being.[2-5] However, recent studies have demonstrated that long-term endurance exercise increases the risk of AF, both in athletes training at a competitive level and in individuals who participate in vigorous exercise at a non-competitive level[6-24, Table 1]. The effects of exercise on the onset and progression of AF is complex and remain unclear with some studies also demonstrating no increased risk of AF with exercise.[25-31] This review focuses on the pathogenesis, clinical presentation, evaluation and management of AF in athletes.
Table 1. Selected Controlled Studies of the Prevalence of AF in Athletes.
| Study Type | Number of subjects | Age/Gender | Type of exercise | Prevalence of AF in athletes/controls (%) |
|---|---|---|---|---|
| Karjalainen et al[15] | 795 | 35-39 years/ Male | Cross country Running | 5.3/0.9 |
| Baldesberger et al[17] | 196 | ~66 years/ Male | Cyclists vs. golfers | 10/0 |
| Mont et al[8] | 216 | <65 years/ Male + Female | Endurance athletes | 63/15 |
| Elosua et al[16] | 109 | 41-55 years/ Male | Endurance athletes | 32/14 |
| Heidbuchel et al[22] | 60 years/83% Male, 17% Female | Cycling, running, or swimming | ||
| Molina et al[18] | 557 | 48 years | Marathon runners vs. sedentary | 5/0.7 |
| Grimsmo et al[11] | 78 | 54-62 years- Group I 72-80 years- Group II 87-92 years- Group III | Cross-country runners, skiers | 12.8 |
Type of Sport and Atrial Fibrillation
Studies have demonstrated that athletes who engage in endurance sports such as runners, cyclists and skiers are more prone to AF than other athletes.[15-18,22,23,32] The exact mechanism involved remains unclear as other athletes who participate in boxing, wrestling, weight-lifting also practice strenuous sport practices, but AF does not appear to be as prevalent in those groups. The question remains – is this related to the type of sport? Unfortunately, little information exists relating to AF risk and sport specificity. Further research is warranted in this area.
Pathophysiology
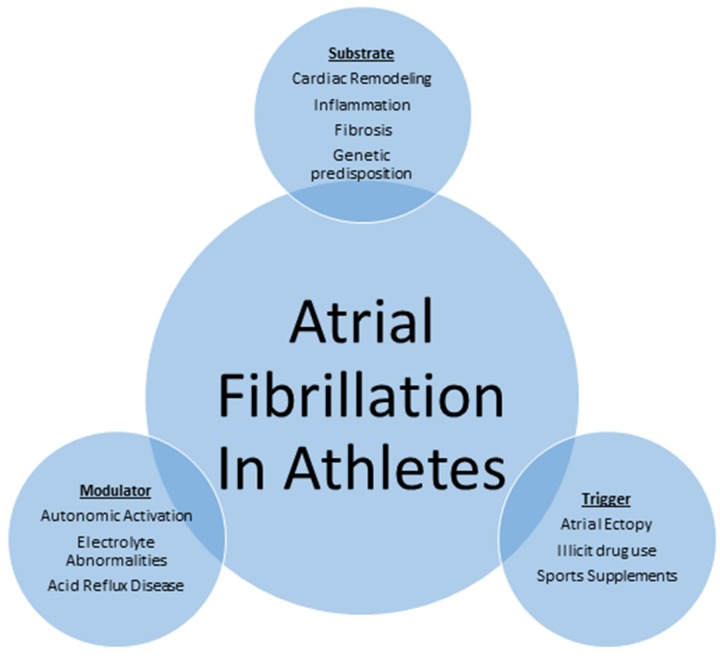
The mechanisms by which exercise contributes to AF are not well understood and are presumed to be multifactorial [Figure 1]. It is accepted that the initiation and perpetuation of AF requires a trigger, a modulator and a substrate. The mechanism of AF in athletes may be attributable to the interaction among these three factors. A specific trigger (atrial ectopy, sports supplements and illicit drug use) in the presence of a suitable substrate (genetic predisposition, inflammation, fibrosis and cardiac remodeling) and a modulator (autonomic activation, electrolyte abnormalities, acid reflux disease) remains the foundation in onset and maintenance of AF in athletes.
Figure 1: Schematic representation of mechanism of atrial fibrillation in athletes.
Triggers of AF
Atrial Ectopy
AF is commonly-triggered by focal ectopic discharges within the four pulmonary veins at the left atrial junction.[33] It has been postulated that increased sympathetic activity when participating in vigorous exercise can trigger atrial ectopy. However, this concept lacks convincing evidence.[13,17,34 ]
A study of 134 professional Swiss athletes and non-athlete controls reported no significant increase in the frequency of isolated atrial ectopic beats (18/62 versus 17/62, p=0.35), despite a significant increase in the frequency AF or atrial flutter (p=0.028) on a 24 hour electrocardiographic monitor.[17]
Sports Supplements
Sports supplements are commonly-consumed by individuals who exercise regularly and indulge in athletic activities, even at a non-competitive level.
Professional athletes occasionally use illicit or performance enhancing drugs, which are not approved by the World Anti-Doping Agency (WADA), to improve performance [Table 2].[35] Anabolic steroids, when associated with competitive sports, has garnered the most attention from media and the public. Increased risk of AF associated with anabolic steroids has been reported in young athletes in isolated case reports.[36,37] There are no systematic data regarding risk of anabolic steroids in the initiation of AF.
Table 2. World Anti-doping Agency (WADA) List of Prohibited Drugs.
| Anabolic Agents |
|
|---|---|
| Beta-2 Agonists | |
| Hormone and Metabolic Modulators |
|
| Diuretics and other masking agents |
|
| Stimulants |
|
| Narcotics |
|
| Cannabanoids | |
| Glucocorticosteroids | |
| Alcohol |
|
| Beta Blockers |
|
Energy drinks have recently gained popularity with young adults participating in competitive and non-competitive sports.[38] Energy drinks contain high levels of caffeine (50 mg to 500mg),[38] stimulants such as taurine, guarana, ginseng, and vitamins such as riboflavin, pantothenic acid and thiamine.[39] Isolated cases of cardiac arrhythmias, including AF with heavy consumption of energy drinks have been recently reported.[38,40] The potential explanation being genetic susceptibility exaggerated by autonomic modulation from high dose caffeine or other ingredients which may trigger AF.
We currently lack objective data on the electrocardiographic effects of energy drinks. One study reported that heavy consumption of energy drinks in healthy individuals can contribute to a transient increase in both blood pressure of 10 mmHg and heart rate of 5-7 beats/minute.[41] However, long-term data concerning the risk of chronic consumption of energy drinks, especially in middle aged individuals who also have other medical comorbidities in triggering AF are lacking.
Substrates for AF
Cardiac Remodeling
There is ample evidence that endurance exercise is associated with both bi-atrial and ventricular enlargement which can occur independent of each other.[8] Left atrial size of >4.0 cm was noted in 20% of athletes who participated in competitive sports.[42] Mont and colleagues reported increased left atrial longitudinal, anteroposterior and transverse diameters and volumes [46.5 ± 17.2 vs 34.6 ± 10.0, p<0.001] with exercise.[19] Atrial enlargement is reported to be related to the lifetime hours of exercise.[43]
Several population studies have demonstrated increased risk of new-onset AF with increased left atrial enlargement.[44,45] However, the role of left atrial remodeling in athletes as a predictor of AF is speculative with no clear evidence. A study of 492-marathon runners with a mean age 42±7 years reported that total higher lifetime hours of training was associated with left atrial enlargement and subsequently higher risk of AF (24% in < 1500 hours, 40% 1500-4500 hours and 83% in > 4500 hours).[13]
Despite increased left atrial size in athletes, one study reported lower left atrial stiffness compared to controls (0.13 ± 0.04 vs. 0.16 ± 0.06, p≤0.01).[46] Another study stratified subjects based on lifetime training as low (<1500 hours), intermediate (1500-4500 hours) and high (>4500 hours). This study reported an increase in left atrial volume (30±5, 33±5 vs. 37±6 ml/m,2 p<0.001), but no effect on atrial mechanical function (pump strain -15.0±2.8, -14.7±2.7 vs. -14.9±2.6%, p=0.92 and conduit strain 23.3±3.9, 22.1±5.3 vs. 23.7±5.7%, p=0.455) measured by two-dimensional echocardiographic speckle track imaging.[47]
Fibrosis
The role of fibrosis in exercise induced-AF mostly from animal models. Sixteen weeks of exercise in Wistar rats substantially increased fibrosis marker expression including fibronectin-1, transforming growth factor-β1, matrix metalloproteinase-2, tissue inhibitor of metalloproteinase-1, procollagen-I, and procollagen-III in the atria and ventricles when compared to controls. Furthermore, exercise cessation reversed fibrosis eight weeks after exercise cessation.[48]
Another study in a similar rat model confirmed the above findings concerning the role of cardiac fibrosis with endurance training by noting an increase in protein major profibrotic markers and messenger RNA synthesis. The study also reported that pretreatment with losartan 50 mg/kg/day reduced all markers of fibrosis.[49]
The role of TNFα-dependent activation of both NFκB and p38MAPK with exercise was recently described in rat models as an underlying mechanism of exercise induced atrial remodeling and AF.[50] However, further research is necessary to examine this mechanism.
Another study including 45 veteran elite athletes and controls demonstrated biochemical evidence of myocardial fibrosis. Markers of collagen turnover including tissue inhibitor of matrix metalloproteinase type I (350 vs. 253 ng/ml, p=0.01); plasma carboxyterminal propeptide of collagen type I (PICP 259 vs. 166 microg/l, p<0.001) and carboxyterminal telopeptide of collagen type I (CITP 5.4 vs. 2.9 microg/l, p<0.001) was demonstrated in veteran athletes when compared to sedentary controls.[51]
In another study including twelve veteran male endurance athletes with a mean age 56±6 years reported that 50% (6/12 athletes) demonstrated evidence of myocardial fibrosis by late gadolinium enhancement (LGE) on cardiac magnetic resonance imaging (CMR). No LGE was demonstrated in sedentary controls. Number of years spent in training (p<0.001) and participation in number of competitive marathons (p<0.001) predicted prevalence of LGE on CMR.[52] Further research is necessary to evaluate the role of cardiac fibrosis with exercise.
Inflammation
The role of inflammation as a substrate in AF is speculative and controversial. Several studies have shown an association between AF and elevated CRP (C-reactive protein).[53,54] Swanson et al hypothesized that excessive training can induce chronic systemic inflammation which may induce high CRP levels that may lead to atrial electrical remodeling and development of AF. Atrial remodeling can be treated with anti-inflammatory drugs.[55] Limited research has demonstrated increased activation of interleukin-6, TNF-α and interleukin beta-1 in induction and maintenance of AF.[56]
Modulators of AF
Autonomic Activation
Several studies have investigated the role of autonomic activation on AF.[12,13,57] AF in athletes is predominantly vagal-mediated.[2] Increased vagal tone initiates AF by creating macro-reentry pathway by increase in the dispersion of the atrial refractory period.[58] Most athletes have a lower resting heart rates which was also a predictor of AF in a study of long-term endurance cross country skiers.[11]
Vagal mediated AF is different from adrenergically-mediated AF which is more commonly seen in the elderly with diseased hearts. Vagal-mediated AF is typically associated with macro-reentry circuits, whereas adrenergically-mediated AF is associated with micro-reentry circuits.[59]
Higher vagal tone was reported in non-elite athletes who participate in regular exercise. A study reported significantly higher vagal tone in non-elite athletes with higher lifetime training hours (>4500 hours versus <1500 hours, 47±16ms vs 34±13ms, p=0.002)].[13]
Mechanistic insight concerning the pathogenesis of AF with exercise comes from animal models. Gausch et al reported that AF duration increased significantly (AF >304 seconds in 64% vs 15%; p < 0.01) in rats who underwent programmed exercise regimen with one hour treadmill training daily for 16 weeks when compared to sedentary controls. Increased vagal tone, atrial dilatation and atrial fibrosis were also reported at 16 weeks in the exercise group. Increased vagal tone was attributed due to messenger ribonucleic acid downregulation of IKACh-inhibiting RGS proteins, was present at 16 weeks in exercising rats. Detraining for 4 weeks normalized vagal tone.[14] The role of IKACh in mediating cardiac response to vagal stimulation was previously described in the genesis of AF from rat models.[60]
Electrolyte Abnormalities
Athletes who are involved in vigorous exercise can have dynamic fluid shifts in the body which can lead to dehydration and alteration in pH and depletion of electrolytes including sodium, potassium and magnesium which may also contribute to AF.
Acid Reflux Disease
Swanson et al postulated that regular exercise can induce gastroesophageal reflux which may induce AF.[57] The association between acid reflux disease, AF and athletes from high vagal tone remains a subject of conjecture.
A study investigated esophageal acidity in healthy athletes and controls after 80 minutes of moderate to hard sprinting. All subjects reported symptoms of acid reflux. Intra-esophageal acidity monitoring was done which showed that in controls pH< 4.0 was noted 4.9% of the time vs. 17.2% of the time for runners fed a light breakfast one hour before the run.[61] Another study reported similar findings of intraesophageal acidity and acid reflux symptoms in 5/11 fasted runners and in 8/9 fed runners during or just after exercise.[62]
Soffer et al, reported a direct correlation between exercise (cycling at a VO2 max of 75% and 90%) and decrease in intra-esophageal pH to below 4.0. In trained cyclists exercising at VO2 max of 75% and 90%, the number of episodes when the pH decreased below 4.0 were 1.2 and 3.7 episodes/hour. In untrained cyclists the number of episodes when the pH decreased was substantially higher at 4.5 and 17.5 episodes/hour respectively.[63,64]
Marathon runners after a 20K race demonstrated evidence of esopghagitis on endoscopy.[65] A large population study including 163,627 patients reported that acid reflux disease increased the risk of AF by 39% [95% confidence interval 1.33-1.45].[66]
Clinical Features and Evaluation
Symptoms such as syncope, palpitations, and dyspnea on exertion reported by athletes should be thoroughly evaluated. A detailed history and a thorough physical examination are warranted. Onset of symptoms with relation to exercise needs to be established. A history of the use of alcohol, sports supplements and energy drinks is important. AF associated in young athletes is usually paroxysmal.
Persistent AF may occur in middle-aged athletes with comorbid cardiovascular conditions. When an athlete reports palpitations it is important to exclude underlying structural heart disease such as arrhythmogenic right ventricular dysplasia, hypertrophic cardiomyopathy, or underlying conduction abnormalities such as the Brugada Syndrome, the Wolff-Parkinson-White syndrome or concealed atrioventricular bypass tracts.
The natural history of vagal-mediated AF in athletes remains unknown. A study of 30 well-trained athletes with a mean age of 48±7 years followed for nine years reported that paroxysmal AF remained stable in half of the athletes and progressed to persistent or permanent AF in relatively few athletes.[21]
For competitive athletes with recurrent episodes of AF, especially if rates are not controlled, symptoms are present, or exercise tolerance is reduced, activity restrictions must be considered. The need to restrict asymptomatic athletes whose ventricular rates are well-controlled is less compelling, but this may depend on the sport.
All athletes with a diagnosis of AF should undergo standard testing such as basic metabolic panel to access for underlying electrolyte abnormalities, a thyroid panel and a transthoracic echocardiography. Cardiac monitoring devices such as a Holter monitor, a cardiac event monitor or rarely, an implantable loop recorder may be used to determine if symptoms correlate with AF episodes.
Management
Treatment of AF in athletes with either rate or rhythm control medications can be challenging. Identification of overtraining in athletes is important as reduction or temporary cessation of exercise may decrease or even prevent AF recurrence. The initial approach should be to recommend reduction of physical activity.
A study of 1772 athletes with a mean follow up of 62 months reported disappearance of AF with detraining.[9] Similarly, Hoogsteen et al[21] reported that sports abstinence improved symptoms of AF in athletes.
Rate control agents such as beta-blockers or calcium channel blockers may decrease performance and should be avoided, if possible, in professional athletes.
Antiarrhythmic drugs represent a reasonable choice in some athletes with AF. Flecainide may be used regularly or as a “pill in the pocket” for athletes with vagal-mediated paroxysmal AF in the absence of structural heart disease. Caution is recommended with flecainide because of the risk of proarrhythmia due to intense adrenergic hypertonia during professional sports.
Disopyramide, a class IA antiarrhythmic drug, was shown to be effective in vagal-mediated and bradycardia-dependent AF. Early small studies with disopyramide in post-cardioversion patients reported maintenance of sinus rhythm in 67% patients at 6 months and 54% at 1 year.[67,68] Disopyramide is poorly-tolerated due to antimuscarinic properties and proarrhtyhmic effects.
Amiodarone a class III antiarrhythmic agent, is a potent rhythm control medication. Caution is required due to long-term toxic effects. There are no studies on the efficacy of angiotensin converting enzyme inhibitors, aldosterone antagonists and statins in vagal-mediated AF related to endurance sports.
The 36th Bethesda Conference[69] recommended that athletes with asymptomatic AF can safely participate in any competitive sports in the absence of structural heart disease with maintenance of appropriate ventricular rate with no decrease in functional capacity when they were in sinus rhythm.
Risk of stroke from AF is calculated by the CHA2DS2-VASc score which assigns 2 points for age >75 years (A2), 1 point for vascular disease (V), 1 point for age 65-74 years (A), and 1 point for female gender in addition to the standard risk factors.[70]
In low risk athletes such as those with a CHA2DS2-VASc score of 0-1, no antithrombotic therapy is recommended. For CHA2DS2-VASc scores of ≥2, oral anticoagulation is recommended with warfarin or one of the novel oral anticoagulants. There are no data regarding the safety of novel oral anticoagulants in athletes with AF. Any form of oral anticoagulation can be a challenge due to increased risk of bleeding with sports activates.
Direct current cardioversion can be considered in athletes with AF lasting <48 hours or under TEE guidance when the duration remains unknown. Athletes returning to exercise participation after cardioversion have a high likelihood of AF recurrence due to increased autonomic hyper-activation.
The value of catheter ablation in vagal AF is emerging, with recent data supporting its role in management of AF in athletes. A study of 20 athletes (mean age 44.4±13.0 years) reported freedom from AF and antiarrhythmic therapy at 36.1 ± 12.7 months with pulmonary vein isolation. This study also reported a significant increase in exercise capacity (from 183 ± 32 to 218 ± 20 W, p < 0.02) and substantial improvement in several quality of life indicators on a self-reported questionnaire.[71] All of the athletes became eligible for competitive sports >6 months after therapy. Another study including 182 subjects undergoing pulmonary vein isolation for AF reported similar arrhythmia-free survival at one year in the lone AF sport group versus controls (59% vs 48%, p=0.44), and similar rates of procedure-related complications (7.1% vs. 4.3%; p=0.45). The frequency of redo pulmonary vein isolation procedures was similar between the lone AF sport group and controls (40.5% vs 37.3%, p=0.5).[72] Koopman et al studied 94 endurance athletes and reported similar AF recurrence after an initial pulmonary vein isolation procedure. However, the recurrence rate was significantly higher in non-endurance controls compared tp endurance athletes (48% vs 34%, p=0.04) after 3 years of follow-up.[73] There was however, similar arrhythmia free survival at 3 years between both groups (87 vs. 85%, p=0.88). The effectiveness of catheter ablation, particularly pulmonary vein isolation, in endurance athletes is partly due to younger age and absence of diseased atria. However, the multifactorial etiology of AF in endurance athletes may be partly responsible for arrhythmia recurrence which may require a repeat procedure. Pulmonary vein isolation should be considered as a first-line therapy in endurance athletes with atrial fibrillation for arrhythmia-free survival, improvement in exercise capacity so that one can resume endurance sports practices. A redo-ablation procedure may be considered with recurrence.
Athletes who are candidates for oral anticoagulation for stroke prevention in AF prior to catheter ablation will likely remain so after the procedure.[74] The issue of cessation of oral anticoagulation in athletes who remain free from AF after catheter ablation is uncertain and requires further study.
Future Directions
There is substantial evidence that endurance training results in exaggerated vagal tone, cardiac remodeling, inflammation and fibrosis, all of which may contribute to the onset of AF in athletes. AF is a major cardiovascular disorder which increases the risk of stroke by 5-fold, mortality by 2-fold and also impairs quality of life and exercise capacity.[72] This disease should not be trivialized as a mere consequence of overtraining.
It has been recently hypothesized that exercise is associated with a U-shaped effect in terms of cardiovascular benefit. Further studies are necessary to examine the specific effects of endurance exercise on AF risk including sport specificity, duration, type, intensity in specific age groups and gender.
Long-term follow-up data regarding chronic effects of intense endurance exercise investigating training modalities and physiological factors (such as heart rate, blood pressure, VO2 max) may help in better understanding of this condition. Research in animal models examining the specific cardiovascular, cellular and molecular adaptation to intense endurance exercise is necessary.
Genetic studies including identification of specific genomes that may be vulnerable with chronic endurance exercise are required.
Several middle-aged endurance athletes may have brief episodes of atrial arrhythmia which can be triggered both increased resting vagal tone and sympathetic activation during intense physical exertion. The question remains as to whether such athletes can safely continue training. Currently, there are no guidelines on how best to manage such arrhythmias in the aging athlete. Pulmonary vein isolation via catheter ablation may be an effective strategy in these patients.
The decisions by the physician is based on clinical judgment and lack clarity of evidence. Decreasing volume and intensity of exercise is recommended. Further investigation with remote cardiac monitoring device may be warranted.
Disclosures
Prior completed study grants from NIH,Squbb,CPI,Geigy,Wyeth-Ayerst,Smith-Kline-Beacchum,Sanofi-Aventis,Astra-Zeneca,St. Jude, Boehringer-Ingelheim.
References
- 1.Turagam Mohit K, Velagapudi Poonam, Kocheril Abraham G. Atrial fibrillation in athletes. Am. J. Cardiol. 2012 Jan 15;109 (2):296–302. doi: 10.1016/j.amjcard.2011.08.041. [DOI] [PubMed] [Google Scholar]
- 2.Mont Lluís, Tamborero David, Elosua Roberto, Molina Irma, Coll-Vinent Blanca, Sitges Marta, Vidal Bárbara, Scalise Andrea, Tejeira Alejandro, Berruezo Antonio, Brugada Josep. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008 Jan;10 (1):15–20. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- 3.Endres Matthias, Gertz Karen, Lindauer Ute, Katchanov Juri, Schultze Jörg, Schröck Helmut, Nickenig Georg, Kuschinsky Wolfgang, Dirnagl Ulrich, Laufs Ulrich. Mechanisms of stroke protection by physical activity. Ann. Neurol. 2003 Nov;54 (5):582–90. doi: 10.1002/ana.10722. [DOI] [PubMed] [Google Scholar]
- 4.Arem Hannah, Moore Steven C, Patel Alpa, Hartge Patricia, Berrington de Gonzalez Amy, Visvanathan Kala, Campbell Peter T, Freedman Michal, Weiderpass Elisabete, Adami Hans Olov, Linet Martha S, Lee I-Min, Matthews Charles E. Leisure time physical activity and mortality: a detailed pooled analysis of the dose-response relationship. JAMA Intern Med. 2015 Jun;175 (6):959–67. doi: 10.1001/jamainternmed.2015.0533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garatachea Nuria, Santos-Lozano Alejandro, Sanchis-Gomar Fabian, Fiuza-Luces Carmen, Pareja-Galeano Helios, Emanuele Enzo, Lucia Alejandro. Elite athletes live longer than the general population: a meta-analysis. Mayo Clin. Proc. 2014 Sep;89 (9):1195–200. doi: 10.1016/j.mayocp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Aizer Anthony, Gaziano J Michael, Cook Nancy R, Manson Joann E, Buring Julie E, Albert Christine M. Relation of vigorous exercise to risk of atrial fibrillation. Am. J. Cardiol. 2009 Jun 1;103 (11):1572–7. doi: 10.1016/j.amjcard.2009.01.374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Abdulla Jawdat, Nielsen Jens Rokkedal. Is the risk of atrial fibrillation higher in athletes than in the general population? A systematic review and meta-analysis. Europace. 2009 Sep;11 (9):1156–9. doi: 10.1093/europace/eup197. [DOI] [PubMed] [Google Scholar]
- 8.Mont Lluís, Elosua Roberto, Brugada Josep. Endurance sport practice as a risk factor for atrial fibrillation and atrial flutter. Europace. 2009 Jan;11 (1):11–7. doi: 10.1093/europace/eun289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Furlanello F, Bertoldi A, Dallago M, Galassi A, Fernando F, Biffi A, Mazzone P, Pappone C, Chierchia S. Atrial fibrillation in elite athletes. J. Cardiovasc. Electrophysiol. 1998 Aug;9 (8 Suppl):S63–8. [PubMed] [Google Scholar]
- 10.Mont L, Sambola A, Brugada J, Vacca M, Marrugat J, Elosua R, Paré C, Azqueta M, Sanz G. Long-lasting sport practice and lone atrial fibrillation. Eur. Heart J. 2002 Mar;23 (6):477–82. doi: 10.1053/euhj.2001.2802. [DOI] [PubMed] [Google Scholar]
- 11.Grimsmo Jostein, Grundvold Irene, Maehlum Sverre, Arnesen Harald. High prevalence of atrial fibrillation in long-term endurance cross-country skiers: echocardiographic findings and possible predictors--a 28-30 years follow-up study. Eur J Cardiovasc Prev Rehabil. 2010 Feb;17 (1):100–5. doi: 10.1097/HJR.0b013e32833226be. [DOI] [PubMed] [Google Scholar]
- 12.Grundvold Irene, Skretteberg Per Torger, Liestøl Knut, Erikssen Gunnar, Engeseth Kristian, Gjesdal Knut, Kjeldsen Sverre E, Arnesen Harald, Erikssen Jan, Bodegard Johan. Low heart rates predict incident atrial fibrillation in healthy middle-aged men. Circ Arrhythm Electrophysiol. 2013 Aug;6 (4):726–31. doi: 10.1161/CIRCEP.113.000267. [DOI] [PubMed] [Google Scholar]
- 13.Wilhelm Matthias, Roten Laurent, Tanner Hildegard, Wilhelm Ilca, Schmid Jean-Paul, Saner Hugo. Atrial remodeling, autonomic tone, and lifetime training hours in nonelite athletes. Am. J. Cardiol. 2011 Aug 15;108 (4):580–5. doi: 10.1016/j.amjcard.2011.03.086. [DOI] [PubMed] [Google Scholar]
- 14.Guasch Eduard, Benito Begoña, Qi Xiaoyan, Cifelli Carlo, Naud Patrice, Shi Yanfen, Mighiu Alexandra, Tardif Jean-Claude, Tadevosyan Artavazd, Chen Yu, Gillis Marc-Antoine, Iwasaki Yu-Ki, Dobrev Dobromir, Mont Lluis, Heximer Scott, Nattel Stanley. Atrial fibrillation promotion by endurance exercise: demonstration and mechanistic exploration in an animal model. J. Am. Coll. Cardiol. 2013 Jul 2;62 (1):68–77. doi: 10.1016/j.jacc.2013.01.091. [DOI] [PubMed] [Google Scholar]
- 15.Karjalainen J, Kujala U M, Kaprio J, Sarna S, Viitasalo M. Lone atrial fibrillation in vigorously exercising middle aged men: case-control study. BMJ. 1998 Jun 13;316 (7147):1784–5. doi: 10.1136/bmj.316.7147.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elosua Roberto, Arquer Andreu, Mont Lluís, Sambola Antonia, Molina Lluís, García-Morán Emilio, Brugada Josep, Marrugat Jaume. Sport practice and the risk of lone atrial fibrillation: a case-control study. Int. J. Cardiol. 2006 Apr 14;108 (3):332–7. doi: 10.1016/j.ijcard.2005.05.020. [DOI] [PubMed] [Google Scholar]
- 17.Baldesberger Sylvette, Bauersfeld Urs, Candinas Reto, Seifert Burkhardt, Zuber Michel, Ritter Manfred, Jenni Rolf, Oechslin Erwin, Luthi Pia, Scharf Christop, Marti Bernhard, Attenhofer Jost Christine H. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur. Heart J. 2008 Jan;29 (1):71–8. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- 18.Molina Lluis, Mont Lluis, Marrugat Jaume, Berruezo Antonio, Brugada Josep, Bruguera Jordi, Rebato Carolina, Elosua Roberto. Long-term endurance sport practice increases the incidence of lone atrial fibrillation in men: a follow-up study. Europace. 2008 May;10 (5):618–23. doi: 10.1093/europace/eun071. [DOI] [PubMed] [Google Scholar]
- 19.Mont Lluís, Tamborero David, Elosua Roberto, Molina Irma, Coll-Vinent Blanca, Sitges Marta, Vidal Bárbara, Scalise Andrea, Tejeira Alejandro, Berruezo Antonio, Brugada Josep. Physical activity, height, and left atrial size are independent risk factors for lone atrial fibrillation in middle-aged healthy individuals. Europace. 2008 Jan;10 (1):15–20. doi: 10.1093/europace/eum263. [DOI] [PubMed] [Google Scholar]
- 20.Hood S, Northcote R J. Cardiac assessment of veteran endurance athletes: a 12 year follow up study. Br J Sports Med. 1999 Aug;33 (4):239–43. doi: 10.1136/bjsm.33.4.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hoogsteen Jan, Schep Goof, Van Hemel Norbert M, Van Der Wall Ernst E. Paroxysmal atrial fibrillation in male endurance athletes. A 9-year follow up. Europace. 2004 May;6 (3):222–8. doi: 10.1016/j.eupc.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Heidbüchel Hein, Anné Wim, Willems Rik, Adriaenssens Bert, Van de Werf Frans, Ector Hugo. Endurance sports is a risk factor for atrial fibrillation after ablation for atrial flutter. Int. J. Cardiol. 2006 Feb 8;107 (1):67–72. doi: 10.1016/j.ijcard.2005.02.043. [DOI] [PubMed] [Google Scholar]
- 23.Myrstad M, Løchen M-L, Graff-Iversen S, Gulsvik A K, Thelle D S, Stigum H, Ranhoff A H. Increased risk of atrial fibrillation among elderly Norwegian men with a history of long-term endurance sport practice. Scand J Med Sci Sports. 2014 Aug;24 (4):e238–44. doi: 10.1111/sms.12150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myrstad Marius, Nystad Wenche, Graff-Iversen Sidsel, Thelle Dag S, Stigum Hein, Aarønæs Marit, Ranhoff Anette H. Effect of years of endurance exercise on risk of atrial fibrillation and atrial flutter. Am. J. Cardiol. 2014 Oct 15;114 (8):1229–33. doi: 10.1016/j.amjcard.2014.07.047. [DOI] [PubMed] [Google Scholar]
- 25.Bapat Aneesh, Zhang Yiyi, Post Wendy S, Guallar Eliseo, Soliman Elsayed Z, Heckbert Susan R, Lima Joao, Bertoni Alain G, Alonso Alvaro, Nazarian Saman. Relation of Physical Activity and Incident Atrial Fibrillation (from the Multi-Ethnic Study of Atherosclerosis). Am. J. Cardiol. 2015 Sep 15;116 (6):883–8. doi: 10.1016/j.amjcard.2015.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pathak Rajeev K, Elliott Adrian, Middeldorp Melissa E, Meredith Megan, Mehta Abhinav B, Mahajan Rajiv, Hendriks Jeroen M L, Twomey Darragh, Kalman Jonathan M, Abhayaratna Walter P, Lau Dennis H, Sanders Prashanthan. Impact of CARDIOrespiratory FITness on Arrhythmia Recurrence in Obese Individuals With Atrial Fibrillation: The CARDIO-FIT Study. J. Am. Coll. Cardiol. 2015 Sep 1;66 (9):985–96. doi: 10.1016/j.jacc.2015.06.488. [DOI] [PubMed] [Google Scholar]
- 27.Kwok Chun Shing, Anderson Simon G, Myint Phyo K, Mamas Mamas A, Loke Yoon K. Physical activity and incidence of atrial fibrillation: a systematic review and meta-analysis. Int. J. Cardiol. 2014 Dec 15;177 (2):467–76. doi: 10.1016/j.ijcard.2014.09.104. [DOI] [PubMed] [Google Scholar]
- 28.Drca Nikola, Wolk Alicja, Jensen-Urstad Mats, Larsson Susanna C. Atrial fibrillation is associated with different levels of physical activity levels at different ages in men. Heart. 2014 Jul;100 (13):1037–42. doi: 10.1136/heartjnl-2013-305304. [DOI] [PubMed] [Google Scholar]
- 29.Gebel Klaus, Ding Ding, Chey Tien, Stamatakis Emmanuel, Brown Wendy J, Bauman Adrian E. Effect of Moderate to Vigorous Physical Activity on All-Cause Mortality in Middle-aged and Older Australians. JAMA Intern Med. 2015 Jun;175 (6):970–7. doi: 10.1001/jamainternmed.2015.0541. [DOI] [PubMed] [Google Scholar]
- 30.Osbak Philip Samuel, Mourier Malene, Kjaer Andreas, Henriksen Jens Henrik, Kofoed Klaus Fuglsang, Jensen Gorm Boje. A randomized study of the effects of exercise training on patients with atrial fibrillation. Am. Heart J. 2011 Dec;162 (6):1080–7. doi: 10.1016/j.ahj.2011.09.013. [DOI] [PubMed] [Google Scholar]
- 31.Ofman Peter, Khawaja Owais, Rahilly-Tierney Catherine R, Peralta Adelqui, Hoffmeister Peter, Reynolds Mathew R, Gaziano J Michael, Djousse Luc. Regular physical activity and risk of atrial fibrillation: a systematic review and meta-analysis. Circ Arrhythm Electrophysiol. 2013 Apr;6 (2):252–6. doi: 10.1161/CIRCEP.113.000147. [DOI] [PubMed] [Google Scholar]
- 32.Andersen Kasper, Farahmand Bahman, Ahlbom Anders, Held Claes, Ljunghall Sverker, Michaëlsson Karl, Sundström Johan. Risk of arrhythmias in 52 755 long-distance cross-country skiers: a cohort study. Eur. Heart J. 2013 Dec;34 (47):3624–31. doi: 10.1093/eurheartj/eht188. [DOI] [PubMed] [Google Scholar]
- 33.Patterson Eugene, Po Sunny S, Scherlag Benjamin J, Lazzara Ralph. Triggered firing in pulmonary veins initiated by in vitro autonomic nerve stimulation. Heart Rhythm. 2005 Jun;2 (6):624–31. doi: 10.1016/j.hrthm.2005.02.012. [DOI] [PubMed] [Google Scholar]
- 34.Bjørnstad H, Storstein L, Meen H D, Hals O. Ambulatory electrocardiographic findings in top athletes, athletic students and control subjects. Cardiology. 1994;84 (1):42–50. doi: 10.1159/000176327. [DOI] [PubMed] [Google Scholar]
- 35.https://wada-main-prod.s3.amazonaws.com/resources/files/wada-2015-prohibited-list-en.pdf . Amazonaws. 0;0:0–0. [Google Scholar]
- 36.Sullivan M L, Martinez C M, Gallagher E J. Atrial fibrillation and anabolic steroids. J Emerg Med. 1999 Sep 28;17 (5):851–7. doi: 10.1016/s0736-4679(99)00095-5. [DOI] [PubMed] [Google Scholar]
- 37.Lau Dennis H, Stiles Martin K, John Bobby, Young Glenn D, Sanders Prashanthan. Atrial fibrillation and anabolic steroid abuse. Int. J. Cardiol. 2007 Apr 25;117 (2):e86–7. doi: 10.1016/j.ijcard.2006.11.199. [DOI] [PubMed] [Google Scholar]
- 38.Seifert Sara M, Schaechter Judith L, Hershorin Eugene R, Lipshultz Steven E. Health effects of energy drinks on children, adolescents, and young adults. Pediatrics. 2011 Mar;127 (3):511–28. doi: 10.1542/peds.2009-3592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McCusker Rachel R, Goldberger Bruce A, Cone Edward J. Caffeine content of energy drinks, carbonated sodas, and other beverages. J Anal Toxicol. 2006 Mar;30 (2):112–4. doi: 10.1093/jat/30.2.112. [DOI] [PubMed] [Google Scholar]
- 40.Di Rocco Jennifer R, During Adelaide, Morelli Peter J, Heyden Marybeth, Biancaniello Thomas A. Atrial fibrillation in healthy adolescents after highly caffeinated beverage consumption: two case reports. J Med Case Rep. 2011;5 () doi: 10.1186/1752-1947-5-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Steinke Leah, Lanfear David E, Dhanapal Vishnuprabha, Kalus James S. Effect of "energy drink" consumption on hemodynamic and electrocardiographic parameters in healthy young adults. Ann Pharmacother. 2009 Apr;43 (4):596–602. doi: 10.1345/aph.1L614. [DOI] [PubMed] [Google Scholar]
- 42.Pelliccia Antonio, Maron Barry J, Di Paolo Fernando M, Biffi Alessandro, Quattrini Filippo M, Pisicchio Cataldo, Roselli Alessandra, Caselli Stefano, Culasso Franco. Prevalence and clinical significance of left atrial remodeling in competitive athletes. J. Am. Coll. Cardiol. 2005 Aug 16;46 (4):690–6. doi: 10.1016/j.jacc.2005.04.052. [DOI] [PubMed] [Google Scholar]
- 43.Pelliccia Antonio, Kinoshita Norimitsu, Pisicchio Cataldo, Quattrini Filippo, Dipaolo Fernando M, Ciardo Roberto, Di Giacinto Barbara, Guerra Emanuele, De Blasiis Elvira, Casasco Maurizio, Culasso Franco, Maron Barry J. Long-term clinical consequences of intense, uninterrupted endurance training in olympic athletes. J. Am. Coll. Cardiol. 2010 Apr 13;55 (15):1619–25. doi: 10.1016/j.jacc.2009.10.068. [DOI] [PubMed] [Google Scholar]
- 44.Fatema Kaniz, Barnes Marion E, Bailey Kent R, Abhayaratna Walter P, Cha Steven, Seward James B, Tsang Teresa S M. Minimum vs. maximum left atrial volume for prediction of first atrial fibrillation or flutter in an elderly cohort: a prospective study. Eur J Echocardiogr. 2009 Mar;10 (2):282–6. doi: 10.1093/ejechocard/jen235. [DOI] [PubMed] [Google Scholar]
- 45.Vaziri S M, Larson M G, Benjamin E J, Levy D. Echocardiographic predictors of nonrheumatic atrial fibrillation. The Framingham Heart Study. Circulation. 1994 Feb;89 (2):724–30. doi: 10.1161/01.cir.89.2.724. [DOI] [PubMed] [Google Scholar]
- 46.D'Ascenzi Flavio, Pelliccia Antonio, Natali Benedetta Maria, Cameli Matteo, Andrei Valentina, Incampo Eufemia, Alvino Federico, Lisi Matteo, Padeletti Margherita, Focardi Marta, Bonifazi Marco, Mondillo Sergio. Increased left atrial size is associated with reduced atrial stiffness and preserved reservoir function in athlete's heart. Int J Cardiovasc Imaging. 2015 Apr;31 (4):699–705. doi: 10.1007/s10554-015-0600-7. [DOI] [PubMed] [Google Scholar]
- 47.Brugger Nicolas, Krause René, Carlen Frederik, Rimensberger Caroline, Hille Ron, Steck Hélène, Wilhelm Matthias, Seiler Christian. Effect of lifetime endurance training on left atrial mechanical function and on the risk of atrial fibrillation. Int. J. Cardiol. 2014 Jan 1;170 (3):419–25. doi: 10.1016/j.ijcard.2013.11.032. [DOI] [PubMed] [Google Scholar]
- 48.Benito Begoña, Gay-Jordi Gemma, Serrano-Mollar Anna, Guasch Eduard, Shi Yanfen, Tardif Jean-Claude, Brugada Josep, Nattel Stanley, Mont Lluis. Cardiac arrhythmogenic remodeling in a rat model of long-term intensive exercise training. Circulation. 2011 Jan 4;123 (1):13–22. doi: 10.1161/CIRCULATIONAHA.110.938282. [DOI] [PubMed] [Google Scholar]
- 49.Gay-Jordi Gemma, Guash Eduard, Benito Begoña, Brugada Josep, Nattel Stanley, Mont Lluís, Serrano-Mollar Anna. Losartan prevents heart fibrosis induced by long-term intensive exercise in an animal model. PLoS ONE. 2013;8 (2) doi: 10.1371/journal.pone.0055427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Aschar-Sobbi Roozbeh, Izaddoustdar Farzad, Korogyi Adam S, Wang Qiongling, Farman Gerrie P, Yang FengHua, Yang Wallace, Dorian David, Simpson Jeremy A, Tuomi Jari M, Jones Douglas L, Nanthakumar Kumaraswamy, Cox Brian, Wehrens Xander H T, Dorian Paul, Backx Peter H. Increased atrial arrhythmia susceptibility induced by intense endurance exercise in mice requires TNFα. Nat Commun. 2015;6 () doi: 10.1038/ncomms7018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lindsay M Mitchell, Dunn Francis G. Biochemical evidence of myocardial fibrosis in veteran endurance athletes. Br J Sports Med. 2007 Jul;41 (7):447–52. doi: 10.1136/bjsm.2006.031534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wilson M, O'Hanlon R, Prasad S, Deighan A, Macmillan P, Oxborough D, Godfrey R, Smith G, Maceira A, Sharma S, George K, Whyte G. Diverse patterns of myocardial fibrosis in lifelong, veteran endurance athletes. J. Appl. Physiol. 2011 Jun;110 (6):1622–6. doi: 10.1152/japplphysiol.01280.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chung M K, Martin D O, Sprecher D, Wazni O, Kanderian A, Carnes C A, Bauer J A, Tchou P J, Niebauer M J, Natale A, Van Wagoner D R. C-reactive protein elevation in patients with atrial arrhythmias: inflammatory mechanisms and persistence of atrial fibrillation. Circulation. 2001 Dec 11;104 (24):2886–91. doi: 10.1161/hc4901.101760. [DOI] [PubMed] [Google Scholar]
- 54.Li Tong, Sun Ze-Lin, Xie Qi-Ying. Meta-analysis Identifies Serum C-Reactive Protein as an Indicator of Atrial Fibrillation Risk After Coronary Artery Bypass Graft. Am J Ther. 2015 Apr 21; () doi: 10.1097/MJT.0000000000000255. [DOI] [PubMed] [Google Scholar]
- 55.Swanson Don R. Atrial fibrillation in athletes: implicit literature-based connections suggest that overtraining and subsequent inflammation may be a contributory mechanism. Med. Hypotheses. 2006;66 (6):1085–92. doi: 10.1016/j.mehy.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 56.Korantzopoulos Panagiotis, Kolettis Theofilos, Siogas Kostas, Goudevenos John. Atrial fibrillation and electrical remodeling: the potential role of inflammation and oxidative stress. Med. Sci. Monit. 2003 Sep;9 (9):RA225–9. [PubMed] [Google Scholar]
- 57.Swanson Don R. Running, esophageal acid reflux, and atrial fibrillation: a chain of events linked by evidence from separate medical literatures. Med. Hypotheses. 2008 Aug;71 (2):178–85. doi: 10.1016/j.mehy.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bettoni Marco, Zimmermann Marc. Autonomic tone variations before the onset of paroxysmal atrial fibrillation. Circulation. 2002 Jun 11;105 (23):2753–9. doi: 10.1161/01.cir.0000018443.44005.d8. [DOI] [PubMed] [Google Scholar]
- 59.Coumel P. Paroxysmal atrial fibrillation: a disorder of autonomic tone? Eur. Heart J. 1994 Apr;15 Suppl A ():9–16. doi: 10.1093/eurheartj/15.suppl_a.9. [DOI] [PubMed] [Google Scholar]
- 60.Kovoor P, Wickman K, Maguire C T, Pu W, Gehrmann J, Berul C I, Clapham D E. Evaluation of the role of I(KACh) in atrial fibrillation using a mouse knockout model. J. Am. Coll. Cardiol. 2001 Jun 15;37 (8):2136–43. doi: 10.1016/s0735-1097(01)01304-3. [DOI] [PubMed] [Google Scholar]
- 61.Collings Kimberly L, Pierce Pratt F, Rodriguez-Stanley Sheila, Bemben Michael, Miner Philip B. Esophageal reflux in conditioned runners, cyclists, and weightlifters. Med Sci Sports Exerc. 2003 May;35 (5):730–5. doi: 10.1249/01.MSS.0000064937.99001.56. [DOI] [PubMed] [Google Scholar]
- 62.Yazaki E, Shawdon A, Beasley I, Evans D F. The effect of different types of exercise on gastro-oesophageal reflux. Aust J Sci Med Sport. 1996 Dec;28 (4):93–6. [PubMed] [Google Scholar]
- 63.Soffer E E, Wilson J, Duethman G, Launspach J, Adrian T E. Effect of graded exercise on esophageal motility and gastroesophageal reflux in nontrained subjects. Dig. Dis. Sci. 1994 Jan;39 (1):193–8. doi: 10.1007/BF02090082. [DOI] [PubMed] [Google Scholar]
- 64.Soffer E E, Merchant R K, Duethman G, Launspach J, Gisolfi C, Adrian T E. Effect of graded exercise on esophageal motility and gastroesophageal reflux in trained athletes. Dig. Dis. Sci. 1993 Feb;38 (2):220–4. doi: 10.1007/BF01307538. [DOI] [PubMed] [Google Scholar]
- 65.Choi S C, Choi S J, Kim J A, Kim T H, Nah Y H, Yazaki E, Evans D F. The role of gastrointestinal endoscopy in long-distance runners with gastrointestinal symptoms. Eur J Gastroenterol Hepatol. 2001 Sep;13 (9):1089–94. doi: 10.1097/00042737-200109000-00016. [DOI] [PubMed] [Google Scholar]
- 66.Kunz Jeffrey S, Hemann Brian, Edwin Atwood J, Jackson Jeffrey, Wu Timothy, Hamm Carolyn. Is there a link between gastroesophageal reflux disease and atrial fibrillation? Clin Cardiol. 2009 Oct;32 (10):584–7. doi: 10.1002/clc.20660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Crijns HJ, Gosselink AT, Lie KI. Propafenone versus disopyramide for maintenance of sinus rhythm after electrical cardioversion of chronic atrial fibrillation: a randomized, double-blind study. PRODIS Study Group. Cardiovascular drugs and therapy / sponsored by the International Society of Cardiovascular Pharmacotherapy. 1996;10:145–152. doi: 10.1007/BF00823592. [DOI] [PubMed] [Google Scholar]
- 68.Karlson B W, Torstensson I, Abjörn C, Jansson S O, Peterson L E. Disopyramide in the maintenance of sinus rhythm after electroconversion of atrial fibrillation. A placebo-controlled one-year follow-up study. Eur. Heart J. 1988 Mar;9 (3):284–90. doi: 10.1093/oxfordjournals.eurheartj.a062498. [DOI] [PubMed] [Google Scholar]
- 69.Maron Barry J, Zipes Douglas P. Introduction: eligibility recommendations for competitive athletes with cardiovascular abnormalities-general considerations. J. Am. Coll. Cardiol. 2005 Apr 19;45 (8):1318–21. doi: 10.1016/j.jacc.2005.02.006. [DOI] [PubMed] [Google Scholar]
- 70.Lip Gregory Y H, Frison Lars, Halperin Jonathan L, Lane Deirdre A. Identifying patients at high risk for stroke despite anticoagulation: a comparison of contemporary stroke risk stratification schemes in an anticoagulated atrial fibrillation cohort. Stroke. 2010 Dec;41 (12):2731–8. doi: 10.1161/STROKEAHA.110.590257. [DOI] [PubMed] [Google Scholar]
- 71.Furlanello Francesco, Lupo Pierpaolo, Pittalis Mario, Foresti Sara, Vitali-Serdoz Laura, Francia Pietro, De Ambroggi Guido, Ferrero Paolo, Nardi Stefano, Inama Giuseppe, De Ambroggi Luigi, Cappato Riccardo. Radiofrequency catheter ablation of atrial fibrillation in athletes referred for disabling symptoms preventing usual training schedule and sport competition. J. Cardiovasc. Electrophysiol. 2008 May;19 (5):457–62. doi: 10.1111/j.1540-8167.2007.01077.x. [DOI] [PubMed] [Google Scholar]
- 72.Calvo Naiara, Mont Lluís, Tamborero David, Berruezo Antonio, Viola Graziana, Guasch Eduard, Nadal Mercè, Andreu David, Vidal Barbara, Sitges Marta, Brugada Josep. Efficacy of circumferential pulmonary vein ablation of atrial fibrillation in endurance athletes. Europace. 2010 Jan;12 (1):30–6. doi: 10.1093/europace/eup320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Koopman Pieter, Nuyens Dieter, Garweg Christophe, La Gerche Andre, De Buck Stijn, Van Casteren Lieve, Alzand Becker, Willems Rik, Heidbuchel Hein. Efficacy of radiofrequency catheter ablation in athletes with atrial fibrillation. Europace. 2011 Oct;13 (10):1386–93. doi: 10.1093/europace/eur142. [DOI] [PubMed] [Google Scholar]
- 74.Go A S, Hylek E M, Phillips K A, Chang Y, Henault L E, Selby J V, Singer D E. Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA. 2001 May 9;285 (18):2370–5. doi: 10.1001/jama.285.18.2370. [DOI] [PubMed] [Google Scholar]