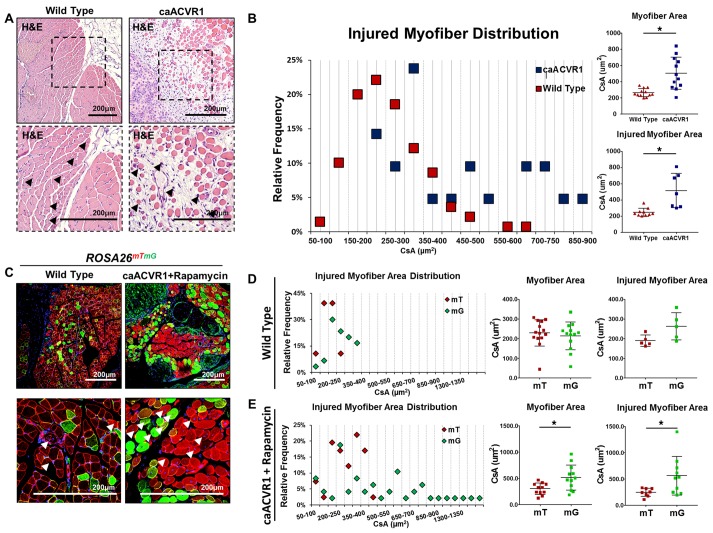
Figure 4. Rapamycin after injury reveals improved myofiber size with hyperactive BMP signaling.
(A) H&E showing areas of myofiber injury in wild-type and caAcvr1fl/fl mice 20 days after adenoviral Cre and cardiotoxin (Ad.cre/CTX) injury (black arrowheads indicate myofibers with centralized nuclei). (B) Distribution of myofiber cross-sectional areas showing significantly increased myofiber cross-sectional area in caAcvr1fl/fl mice when compared with wild-type mice 20 days after Ad.cre/CTX injury (503 vs. 264 μm2 for all myofibers, 551 vs. 251 μm2 for centrally nucleated myofibers, Student’s 2-tailed t test, n = 4 high-powered fields (HPFs) from 3 distinct biological samples, P < 0.05). (C) Representative fluorescence images of wild-type (ROSA26mTmG) and mutant (caAcvr1fl/fl ROSA26mTmG) mice 20 days after Ad.cre/CTX injury and either no treatment (wild-type) or rapamycin treatment (mutant). (D) Distribution of green and red myofiber cross-sectional area (μm2) in wild-type ROSA26mTmG mice 20 days after Ad.cre/CTX injury showing absence of statistically significant or substantial difference in cross-sectional area (227 vs. 243 μm2, Student’s 2-tailed t test, n = 4 HPFs from 3 distinct biological samples, P = not significant). (E) Distribution of green and red myofiber cross-sectional area (μm2) in caAcvr1fl/fl ROSA26mTmG mice 20 days after Ad.cre/CTX injury showing significantly increased green myofiber cross-sectional area (519 vs. 305 μm2 for all myofibers, 551 vs. 251 μm2 for injured myofibers, Student’s 2-tailed t test, n > 7 HPFs from 3 distinct biological samples, P < 0.05). *P < 0.05. All scale bars: 200 μm. CsA, cross-sectional area; mT, membrane-bound RFP; mG, membrane-bound GFP.