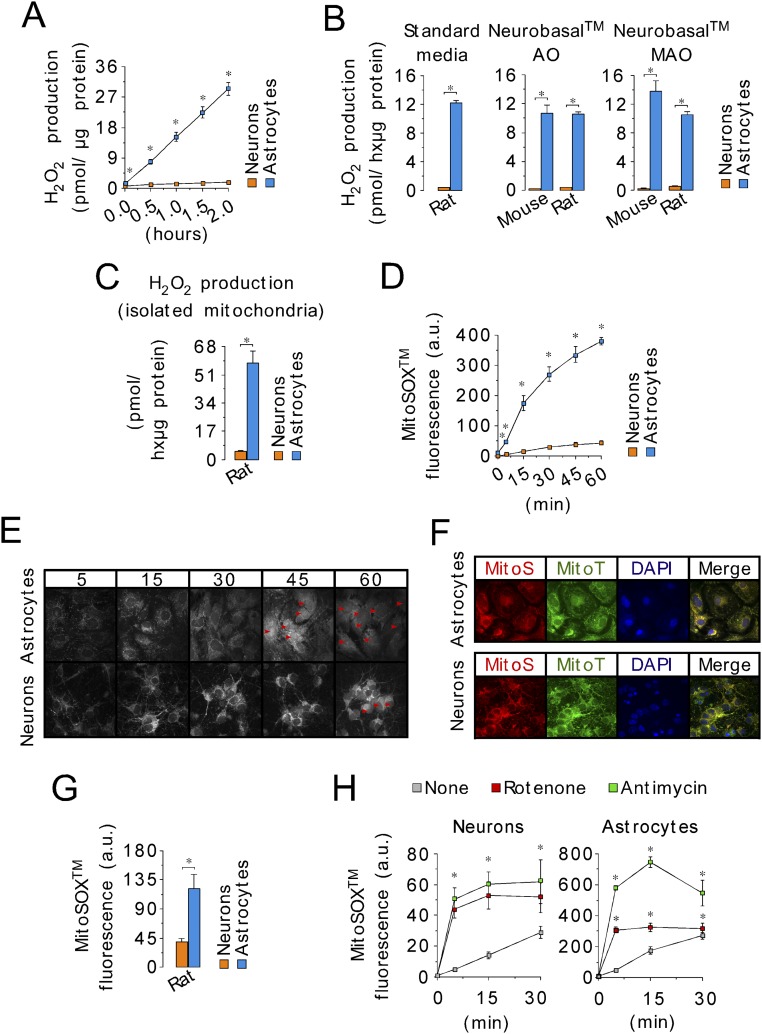
Fig. S2.
The higher ROS production in astrocytes compared with neurons is conserved in mouse and rat and is not dependent on cell culture conditions. (A) A kinetics analysis of H2O2 production in neurons and astrocytes from C57BL/6 mice assayed by AmplexRed shows linearity with time up to 2 h. (B) Rates of H2O2 production, as assessed using the AmplexRed assay, in intact C56BL/6 mouse or Wistar rat neurons and astrocytes in primary culture, after replacement of the standard medium with antioxidants (standard medium or Neurobasal AO) or without antioxidant (Neurobasal MAO) for the last 24 h. (C) Rates of H2O2 production assessed using the AmplexRed assay, in mitochondria isolated from Wistar rat neurons and astrocytes in primary culture. (D) Mitochondrial ROS as quantified using the MitoSox assay in the intact cells by flow cytometry at different times. (E) Confocal images of neurons and astrocytes loaded with MitoSox showing mitochondrial-like localization of the probe at 5, 15, and 30 min, but both mitochondrial-like and nuclear (red arrows) localization at 45 and 60 min. Accordingly, MitoSox fluorescence was evaluated at 30 min (or 15 min) in all subsequent experiments. (Magnification: 40×.) (F) To confirm mitochondrial, but not nuclear, MitoSox localization at 30 min, confocal images of cultured mouse astrocytes and neurons were performed to show the colocalization of MitoSox (MitoS) with the mitochondrial-tagged dye Cytopainter (MitoT). (Magnification: 40×.) DAPI was used to stain nuclei. (G) Mitochondrial ROS as quantified using the MitoSox assay at 30 min in intact cells (Wistar rats) by flow cytometry. (H) Quantification of MitoSox fluorescence in neurons and astrocytes in primary culture in the absence (none) or presence of complex I (rotenone, 10 µM) or complex III (antimycin, 10 µM) inhibitors, at 5-, 15-, and 30-min reveals that 15 min is sufficient to achieve maximal increase in mitochondrial ROS. Data are the mean values ± SEM from n = 3–4 independent culture preparations (Student’s t test). *P < 0.05.