Significance
We have developed a method for finding pharmaceuticals that would treat obesity and type 2 diabetes by increasing the metabolic rate of resting skeletal muscle. The metabolic rate is increased by shifting the motor protein myosin from a low activity state to a higher activity state. We devised an assay, screened for compounds, and found one molecule, piperine. Piperine increased the metabolic rate of resting muscle fibers. Piperine does not have the properties required to be a pharmaceutical in humans, but it would make a good lead compound for finding compounds that do. Our results provide proof of concept that these metabolic diseases can be treated by future pharmaceuticals that target myosin to increase the metabolism of excess calories.
Keywords: myosin, fluorescence, skeletal muscle, super-relaxed state, obesity
Abstract
We identify a target for treating obesity and type 2 diabetes, the consumption of calories by an increase in the metabolic rate of resting skeletal muscle. The metabolic rate of skeletal muscle can be increased by shifting myosin heads from the super-relaxed state (SRX), with a low ATPase activity, to a disordered relaxed state (DRX), with a higher ATPase activity. The shift of myosin heads was detected by a change in fluorescent intensity of a probe attached to the myosin regulatory light chain in skinned skeletal fibers, allowing us to perform a high-throughput screen of 2,128 compounds. The screen identified one compound, which destabilized the super-relaxed state, piperine (the main alkaloid component of black pepper). Destabilization of the SRX by piperine was confirmed by single-nucleotide turnover measurements. The effect was only observed in fast twitch skeletal fibers and not in slow twitch fibers or cardiac tissues. Piperine increased ATPase activity of skinned relaxed fibers by 66 ± 15%. The Kd was ∼2 µM. Piperine had little effect on the mechanics of either fully active or resting muscle fibers. Previous work has shown that piperine can mitigate both obesity and type 2 diabetes in rodent models of these conditions. We propose that the increase in resting muscle metabolism contributes to these positive effects. The results described here show that up-regulation of resting muscle metabolism could treat obesity and type 2 diabetes and that piperine would provide a useful lead compound for the development of these therapies.
An epidemic of obesity and type 2 diabetes is currently affecting a large fraction of the world population (1, 2). This is primarily due to an overconsumption of food coupled with a reduction in physical activity. A natural antidote to both obesity and type 2 diabetes is to choose a healthy lifestyle, including a low-calorie diet and increased physical activity. However, many do not choose this option, and for some advanced patients, increased physical activity is not possible. The response to overfeeding in humans is diverse: some store the excess calories almost entirely, whereas others metabolize most of them (3). The variation is strongly dependent on both lifestyle and genetics. As strenuous activity was limited in some of these protocols, the diversity of weight gain was attributed to light activities, such as fidgeting (3, 4). Alternatively, the diversity may be due to variation in the metabolic rate of resting muscle and to how this responds to activity (5).
A pharmacological approach to combating obesity has been of limited value, of the order of 5–10% weight loss when combined with lifestyle changes (for review, see refs. 6 and 7). Therapies for type 2 diabetes produce little effect, with the exception of metformin (for review, see ref. 8). Lifestyle changes, including better diets and increased activity levels, have a larger effect than pharmaceuticals. Here we suggest a target for combating obesity and type 2 diabetes, increasing the metabolic rate of resting skeletal muscle. The metabolic rate of resting, living skeletal muscle is variable, responding to a number of factors, including the hormones leptin and epinephrine (for review, see ref. 5). Although the resting metabolism of muscle is low compared with many other tissues, due to its large mass, ∼40% of body weight, its contribution to whole-body resting metabolic rate is appreciable, ∼25%. This strategy will address the fundamental problem in these conditions: fat and glucose intake exceed the amount consumed by metabolism. A common problem following weight loss is regain of weight due to a reduction in metabolic rate (9). A pharmaceutical that increases metabolic rate may help achieve weight loss and maintain it. Skeletal muscle is an ideal tissue for increasing thermogenesis because of its large metabolic capacity. The resting metabolic rate is ∼15W (for review, see ref. 5). Human subjects can raise the metabolic rate to 600 W for 1 h (10, 11).
We have recently identified a new mechanism for thermogenesis in resting muscle, variation in the ATPase activity of the motor protein myosin (5, 12, 13). Measuring single-cycle ATP turnover rates in skinned resting muscle, we showed that myosin has two states, one with a turnover time of ∼20 s, similar to the rate for purified myosin at 25 °C, and one with a turnover time of about 250 s. It had been shown many years earlier by a comparison between the in vivo rate of resting muscle and the activity of purified myosin that the myosin ATPase activity in resting skeletal fibers was highly inhibited (14). A correlation with structural data suggested that the myosin heads with the slow turnover time were bound to the core of the thick filament in a complex known as the interacting-heads motif (12, 15–17). The myosin heads with the faster ATPase activity are not bound to the thick filament and are disordered and capable of binding weakly to actin in resting fibers. We have called the myosin state with the slower ATPase activity the super-relaxed state (SRX) and the one with the faster ATPase activity the disordered relaxed state (DRX) (5, 12, 13). The low metabolic rate of resting skeletal muscle requires that myosin heads spend most of their time in the SRX in both animals and in humans. A simple calculation shows that if all myosin heads were transferred from the SRX to the DRX, human whole-body metabolic rate would increase by ∼2–4 MJ · d‒1 (5). Thus, a pharmaceutical that destabilized a large fraction of myosin in the SRX would be an effective therapy for obesity and type 2 diabetes.
A major hurdle to discovering small molecules that destabilize the SRX has been the limitations imposed by the single-nucleotide turnover assay previously used and the requirement of observing it in fibers. We recently overcame these obstacles by finding a fluorescent probe on a subunit of myosin, the regulatory light chain (RLC), which showed an increase in emission intensity and shift to shorter wavelengths upon the transition from the SRX to the DRX (Figs. S1 and S2) (18). We used this signal to carry out a high-throughput screen of 2,128 compounds approved for human consumption by the FDA (see Supporting Information for description). This screen identified one compound, piperine, the main alkaloid component of black pepper.
Fig. S1.
Image of one of the wells, showing the chopped, skinned fibers labeled with MDCC. The fiber suspension was pipetted into a well in a 384-well plate, and the fibers were allowed to settle to the bottom of the well. The well was imaged using epifluorescence with a Nikon 6D microscope. The fluorescence intensity of the fibers was then quantitated by a macro and used to measure the population of myosin heads in the SRX.
Fig. S2.
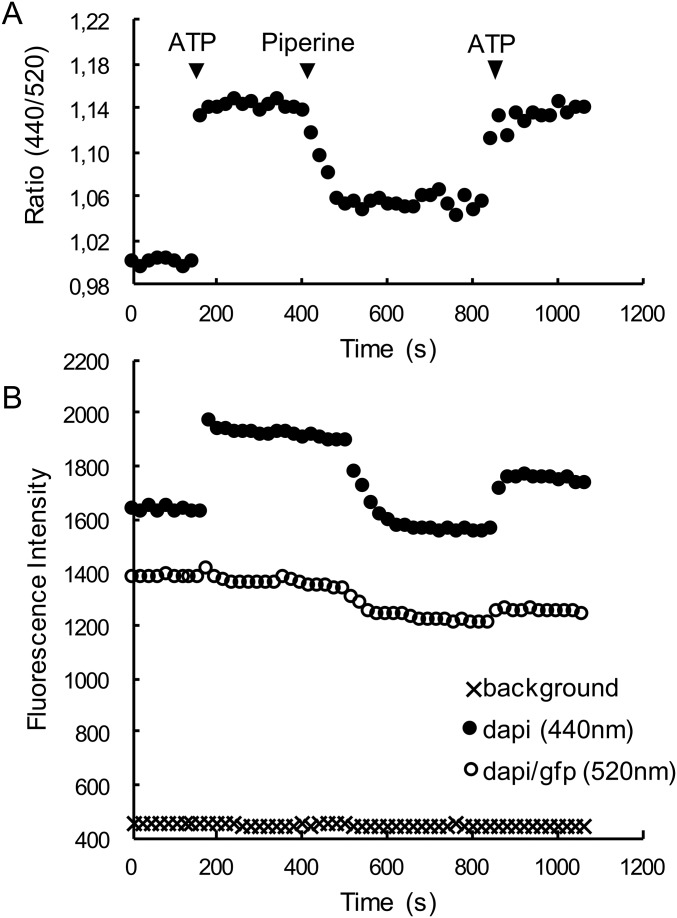
Fluorescence signals for the flow-cell experiment shown in Fig. 1. (A) The ratio of the DAPI channel (emission = 440 nm) to the DAPI/GFP channel (emission = 520 nm) is shown as a function of time. (B) The fluorescence signals for the DAPI channel (closed circles) and the DAPI/GFP channel (open circles). Also shown is the background for the DAPI channel (crosses), which is similar to that for DAPI/GFP. The addition of ATP caused the intensity of the DAPI channel to increase more than the DAPI/GFP channel due to the blue shift of the RLC-C5-MDCC signal. Upon addition of 100 µM piperine the intensity of the DAPI channel decreased significantly, with a smaller decrease in the DAPI/GFP channel. Upon addition of ATP to wash out piperine both signals increase. Note that the two fluorescence signals decrease slowly due to photo bleaching, whereas the ratio remains steady. Although piperine has a weak fluorescence it does not contribute to these signals, as shown by the lack of change in the background upon addition of piperine. The fluorescence of an unlabeled fiber was less than 10 units and was unchanged in the presence of either ATP or piperine.
Results
The Signal Used in the Screen.
Our previous work had identified the ratio of fluorescent intensities as a reporter of the population of the SRX (18). We used an RLC mutant labeled with a coumarin maleimide (MDCC) on cysteine 5. The emission of the probe shifted to lower wavelengths upon the transition from rigor or the DRX to the SRX. The intensity obtained at shorter wavelengths, 440 nm, was divided by that obtained at longer wavelengths, 520 nm, to produce a ratio that was intrinsic to the state of myosin and independent of the number of fibers being visualized, intensity of excitation, and photobleaching.
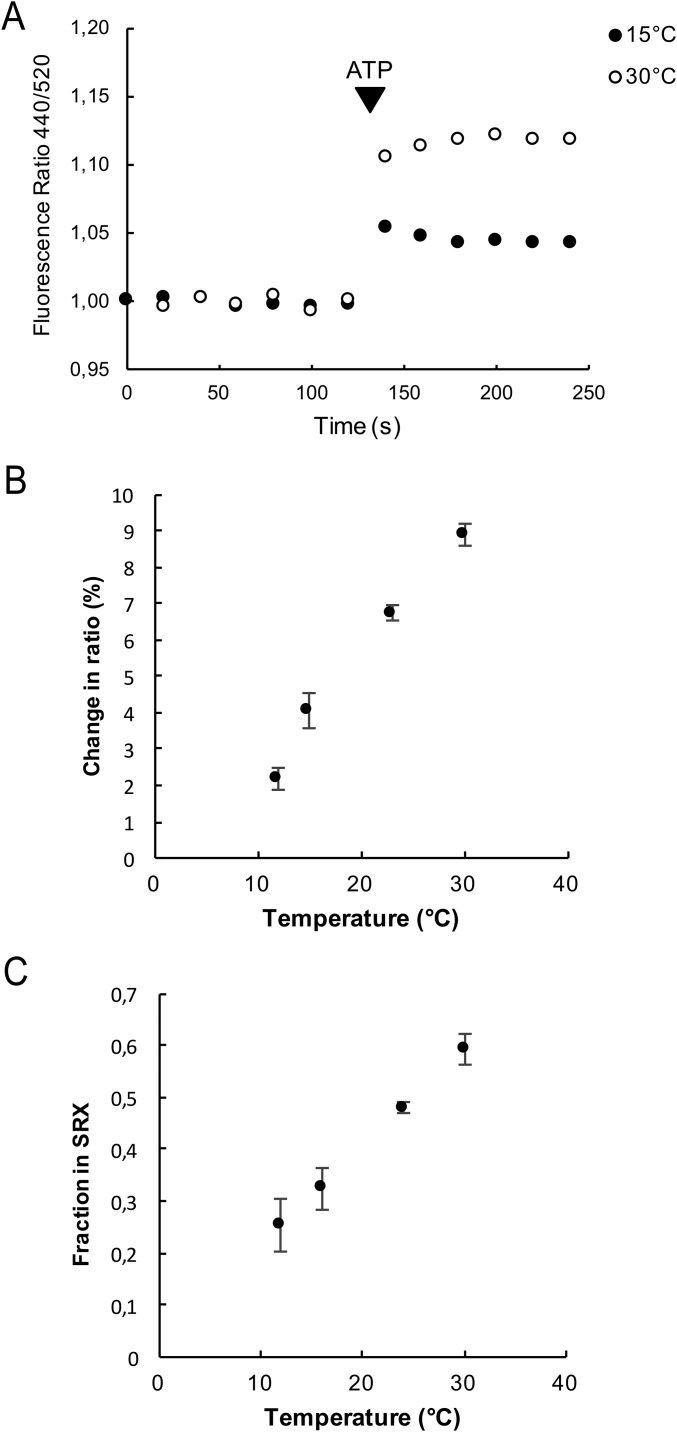
To strengthen the hypothesis that this signal reports the population of the SRX, we measured the population of the SRX by the ratio signal at different temperatures. The population of the SRX measured by the mant–chase protocol and the binding of myosin heads to the core of the thick filament, measured by X-ray diffraction, have both shown a strong temperature dependence (12, 19). As shown in Fig. S3, the population of the SRX measured by the ratio signal also has a strong temperature dependence, increasing by a factor of 2.2 ± 0.2 (SEM, n = 6) on raising the temperature from 15 °C to 30 °C. This change in population is similar to that observed previously using the chase protocol, which found that the population increased by a factor of 1.8 over the same temperature range. These observations provide additional support for the hypothesis that the fluorescence ratio reports the population of the SRX.
Fig. S3.
(A) The ratio of the intensities of the fluorescent emission of a fiber exchanged with RLC-C5-MDCC is shown as a function of time at 15 °C and 30 °C. The fiber starts in rigor, and ATP is added as indicated. The increase in the ratio upon addition of ATP is 4.9% at 15 °C and 12.2% at 30 °C. This observation shows that the population of the SRX, measured here by the ratio signal, has increased by 2.2-fold. This is similar to the change in the population of the SRX measured previously by the mant–nucleotide chase experiment, which increased by 1.8-fold with the same temperature change (12). This observation provides support for the hypothesis that the ratio signal responds to the formation of the SRX and not simply to the binding of nucleotides. Note, the change in the ratio on addition of ATP is smaller in this preparation than for the one shown in Fig. 1, probably due to less specific exchange of the labeled RLC. The data have been normalized so that each rigor ratio is one for comparison. (B) The change in the ratio of fluorescence intensities upon the transition from rigor to relaxed is plotted as a function of temperature. (C) The fraction of myosin heads in the SRX, measured by the mant–chase experiment, is shown as a function of temperature, taken from Stewart et al. (12). As can be seen by comparison with B, the temperature dependence is similar to that measured by the ratio of fluorescence intensities.
High-Throughput Screen.
The screens were carried out by observing the change in fluorescence intensity of probes attached to a subunit of myosin, RLC, in skinned rabbit fast skeletal muscle fibers. The wells were loaded using the labeled and chopped fiber preparation as described in Materials and Methods and Supporting Information (Fig. S1). Because of the low homogeneity of the fiber preparation, fiber number varied among wells. Even if fibers were loosely attached to the bottom of the well, it was not possible to exclude movements during the assay. To further complicate matters the fluorescent excitation intensity was not uniform across the well. These problems have been addressed by the discovery of a fluorescence ratiometric reporter of the myosin state, described above (18). The images were analyzed at two wavelengths by the Fiji macro, and outliers were reexamined by manual analysis. See Supporting Information for a more detailed description of the fiber preparation and the high-throughput screen.
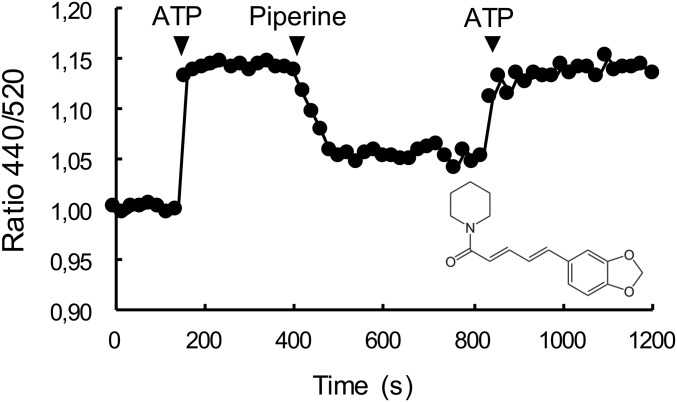
In only one well did the intensity ratio indicate that the SRX of the fibers had been destabilized by the compound present in that well. The compound was piperine, which is the main alkaloid component of black pepper (Fig. 1). Piperine is a well-known compound in ayurvedic medicine and has been shown to interact with a number of targets in biological systems.
Fig. 1.
The effect of 100 μM piperine on the fluorescence of fast skeletal RLC-MDCC fibers. A single labeled fiber was mounted in a simple flow cell, and fluorescence was measured at two wavelengths. The ratio of the two intensities, which measures the population of the myosin heads in the SRX (18), is shown as a function of time. The fiber starts in rigor where the population of the SRX is zero. The ratio increases upon addition of ATP showing an increased population of the SRX. Subsequent addition of 100 μM piperine to the relaxing solution decreases the ratio showing that ∼60% of the myosin heads have transferred out of the SRX into the DRX. A subsequent wash with ATP shows that the effect of piperine is reversible. The structure of piperine is shown in the lower right.
Flow Cells.
To confirm that piperine was a true hit, we observed its effect on a labeled fiber mounted in a flow cell on an inverted fluorescence microscope. This setup allowed the quick exchange of solutions with continuous measurement of the fluorescence emission at both wavelengths. In a typical experiment the fiber started in rigor buffer, and a relaxing solution was added to populate the SRX state (Fig. 1 and Fig. S2). In the presence of ATP, the ratio of the fluorescence intensity increased by about 15%. The solution was replaced by a relaxing solution containing 100 µM piperine, producing about a 10% drop in the intensity ratio and indicating that piperine had destabilized ∼60% of the previously existing myosin in the SRX. The solution was again exchanged with a relaxing solution without piperine, and the ratio returned to its previous level, showing that the effect of piperine was reversible. Data from a number of similar experiments showed that 100 µM piperine destabilized the population of the SRX by 46 ± 3% (SEM, n = 22).
The data from the screen used the fluorescent signal of a labeled subunit of myosin to monitor the population of the SRX. A more definitive experiment is to directly measure the rate of nucleotide turnover in the fibers, using the single-nucleotide turnover experiments that were first used to identify the SRX (12, 13). In these experiments the fiber is first incubated in mantATP, a fluorescent analog of ATP. This is followed by the chase phase, using unlabeled ATP to displace the fluorescent nucleotides as soon as they are released (Fig. S4C). In this experiment, fluorescence decays in two phases: a fast phase involving the release of mant nucleotides from ATPases with rapid turnover rates, followed by a slow phase, which arises from the release of mant nucleotides from myosin in the SRX.
Fig. S4.
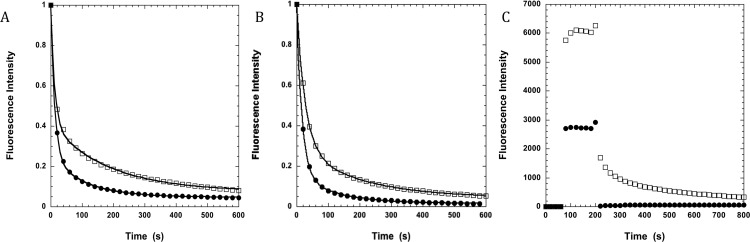
The single-nucleotide turnover experiments used to measure the SRX in skinned skeletal muscle fibers. (A) Control fiber in the presence (solid circles) and absence (open squares) of 100 µM piperine. (B) Fiber exchanged with RLC5-MDCC also in the presence and absence of piperine. (C) The raw data for the control fiber in the absence of piperine showing the fiber fluorescence (open squares) and the background (solid circles). For the control fiber in the absence of piperine the value of P2 is 0.34 and that of T2 is 185. In the presence of piperine, these are inhibited by 41 and 42%, respectively. For the exchanged fiber in the absence of piperine the value of P2 is 0.25 and that of T2 is 194. In the presence of piperine, these are inhibited by 40 and 41%, respectively. The P2 of the exchanged fiber is inhibited relative to the control fiber by 27%, with little change in T2. A similar small change in P2, 0.26 ± 0.02, vs. control, P2 = 0.31, was also seen after exchange with unlabeled RLC-5 by Nogara et al. (18), suggesting that the mutation has a small effect but the addition of the probe causes no further change. In C the fiber starts in rigor, where the fiber fluorescence is indistinguishable from background. The autofluorescence of the fiber was not changed in the presence of ATP or piperine. Upon the addition of 250 μM mantATP at 80 s, both the background and fiber fluorescence increase dramatically. The chase phase begins at 220 s with the wash by 4 mM ATP. The background drops immediately, and fiber fluorescence decreases slowly as mant nucleotides are released and diffuse out of the fiber. In the analysis of the data the background is subtracted from the fiber signal to give net fiber intensity. The net fiber intensity during the chase is then normalized to the value before the chase, providing the curve shown for the control fiber in A.
Typical data for fast skeletal fibers are shown in Fig. 2A, where it is clear that both the magnitude and lifetime of the component of the fluorescence that decays slowly are diminished in the presence of 100 µM piperine. Data obtained in a number of similar experiments showed that piperine destabilized the population of myosin heads that were in the slow component by 34 ± 4% (SEM, n = 10). The lifetime of the heads remaining in the slow component is decreased by 29 ± 11% (SEM, n = 9). In fibers exchanged with RLC-MDCC, there is a small inhibition of the population of the slow component, 27%, but little change in the inhibition caused by piperine (Fig. S4 A vs. B).
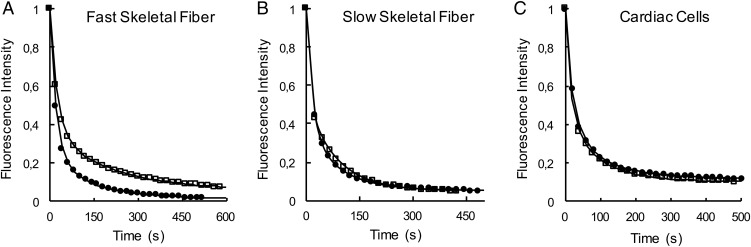
Fig. 2.
The single-nucleotide turnover experiment used to measure the SRX. Single skinned skeletal muscle fibers or strips of cardiac tissue were mounted in a flow cell, incubated in a fluorescent analog of ATP, mantATP, and chased with ATP. The intensity of the fiber fluorescence is plotted as a function of time during the chase phase. Each plot shows samples in the absence (open squares) and presence (solid circles) of 100 μM piperine. Fiber fluorescence decreases in two phases: a fast phase that is largely over in about 20 s, followed by a slow phase with a lifetime of minutes. The slow phase arises from the slow release of nucleotides by myosin in the SRX. (A) Fast twitch skeletal fibers. Data averaged from a number of such experiments showed that populations of the slow fluorescent component were control, 32 ± 3% (SEM, n = 10), piperine, 21 ± 2%, (SEM, n = 18), and the lifetimes were control, 191 ± 13 s (SEM, n = 9), piperine, 136 ± 8 s (SEM, n = 8). Both the population and the lifetime show that the SRX has been partially destabilized. (B) Slow twitch fibers, showing little effect of piperine. Averaged populations of the slow fluorescent component were control, 33 ± 4% (SEM, n = 4), piperine, 33 ± 3% (SEM, n = 8), and the lifetimes were control, 107 ± 10 s (SEM, n = 4), piperine, 98 ± 8 s (SEM, n = 8). (C) Cardiac tissues showing little effect of piperine. Averaged populations of the slow fluorescent component were control, 21 ± 2% (SEM, n = 11), piperine, 21 ± 3% (SEM, n = 13), and the lifetimes were control, 146 ± 24 s (SEM, n = 11), piperine, 178 ± 28 s (SEM, n = 13).
The screen and all of the fiber experiments discussed above were performed using rabbit psoas, a fast twitch muscle predominantly composed of myosin type 2B fibers (20–22). To assess a possible fiber-type specificity, the experiment was repeated using fibers isolated from the soleus muscle, which uses a slow twitch type 1 myosin isoform. In contrast to fast twitch fibers, piperine showed little effect on the SRX in the slow twitch fibers (Fig. 2B). The addition of piperine also showed little effect on the SRX in strips of rabbit cardiac tissue (Fig. 2C). Slow twitch skeletal fibers share the same myosin heavy-chain and regulatory light-chain isoforms as cardiac ventricle (20–22), so it is expected that if piperine targets one of these chains, the results would be similar in the two muscles. These observations have important consequences for the development of piperine as a therapeutic compound. An effect of piperine in cardiac tissue would have been very undesirable in a therapeutic agent targeting skeletal muscle. In addition, if the compound only affects fast twitch fibers in humans, as it does in the rabbit fibers, this would limit its power to elevate thermogenesis.
ATPase Activity.
The data described above show that piperine destabilizes the SRX, shifting a large fraction of its population into the DRX state, where its ATPase activity is about tenfold greater. This destabilization would be expected to increase fiber ATPase activity. To explore this possibility, the ATPase activities of bundles of skinned fibers were measured by the amount of inorganic phosphate produced over time (Fig. 3). In the absence of piperine the fiber ATPase activity is 0.06 s−1 (expressed per head of myosin). This result is within the range of values obtained previously, 0.05–0.1 s−1 (23–25), which has been noted to be too high to be compatible with the low resting metabolic rate of living muscle (24). The addition of 100 µM piperine to the solution bathing the fibers produced a 66 ± 10% increase in the ATPase activity of the fibers.
Fig. 3.
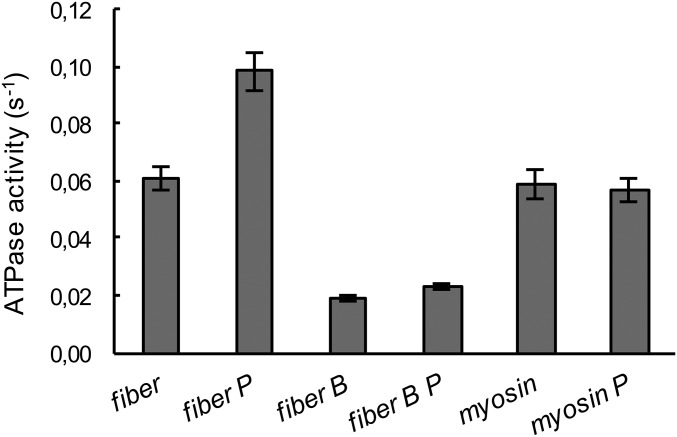
The effect of piperine on the ATPase activity of skinned skeletal muscle fibers and purified myosin. Multiple single fibers were incubated in a small aliquot of relaxing solution, without (column “fiber”) or with 100 μM piperine (column “fiber P”), and their ATPase activity was expressed as the turnover rate per myosin head per second (Fig. S5). Piperine increases the ATPase activity of control fibers by 66 ± 10%. Samples were also run in the presence of 40 μM blebbistatin (columns “fiber B” and “fibers B P” with piperine), which inhibits myosin ATPase activity but not the activity of other enzymes in the fiber, and piperine has little effect on these enzymes. Piperine has a small and not significant effect on the ATPase activity of purified myosin (columns “myosin” and “myosin P”). Together these data show that piperine increases fiber ATPase activity by destabilizing the SRX. Errors are SEM (n = 8–12).
The skinned fiber sample observed here is a complex system, containing a number of other ATPases in addition to myosin. To determine whether piperine affects these activities, we used a specific myosin inhibitor, blebbistatin, which decreases myosin ATPase activity by about 90% (26, 27). The ATPase activity of nonmyosin proteins accounts for about one third of the total, and the small increase seen with piperine may be due to blebbistatin-free myosin heads (Fig. 3, columns 3 and 4). Piperine had little effect on the ATPase activity of purified myosin (Fig. 3, columns 5 and 6). This result suggests that the effect of piperine is specific for the myosin heads in the SRX complex and not to myosin itself. Exchange of fibers with RLC-MDCC did not alter the effect of 100 µM piperine on fiber ATPase activity (Fig. S5). Together, the data show that piperine increases the ATPase activity of skinned fibers by destabilizing the SRX. The fraction of myosin heads involved can be estimated by comparing the increase in fiber activity, ∼0.04 s−1, with that of pure myosin, ∼0.06 s−1. This shows that a large fraction of the myosin heads, >50%, would have to be shifted from the SRX to the DRX to produce the observed increase.
Fig. S5.
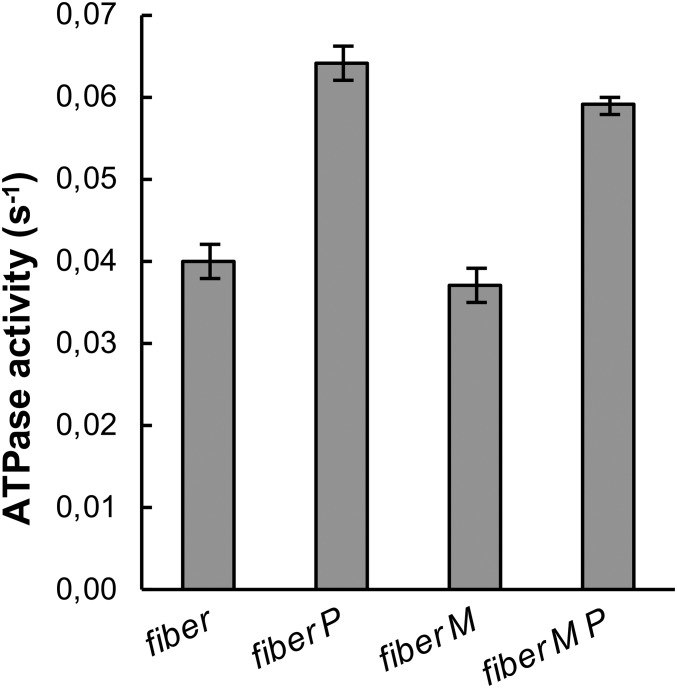
The effect of piperine on the ATPase activity of skinned skeletal muscle fibers before and after exchange with RLC-MDCC. Multiple single fibers were incubated in a small aliquot of relaxing solution, without (column “fiber”) or with 100 μM piperine (column “fiber P”). The ATPase activity was measured using the method described by Lanzetta et al. (50). ATPases were normalized per myosin head by measuring the protein in the fibers using the Bradford assay and, assuming that one half of the protein is myosin, determined by quantitative analysis of gels run in SDS as described in ref. 54. Piperine increases the ATPase activity of control fibers by 60 ± 5%. The experiment was repeated after exchange with RLC-MDCC. Exchange did not alter fiber ATPase activity in the absence of piperine (column “fiber-M”) or in the presence of piperine (column “fiber-M P”). The increase for exchanged fibers in the presence of piperine was 59 ± 5%. Although the basal ATPase activity in this fiber preparation is a little lower than that shown in Fig. 3, 0.04 s−1 vs. 0.06 s−1, the increase in the presence of piperine is similar. Errors are SEM (n = 14–20).
Measuring Affinity.
Estimating the affinity for the binding of piperine to its target is complicated, because piperine is very hydrophobic and binds to the many hydrophobic surfaces available in the interior of the fiber. Nonspecific binding acts in two ways: it decreases the concentration of free piperine inside the fiber and it provides a larger pool of available binding sites that need to be filled. The full effect of piperine requires about 80 s at a concentration of 100 µM (Fig. 1). The time increased with decreasing piperine and reached more than 1 h at 10 μM. We observed chopped fibers in plates, where they were stable for long periods, and we measured ATPase activities after preincubation in relaxing solutions plus piperine, as described in Materials and Methods. In each experiment, data were obtained at a particular concentration and also at a high concentration, 80–100 µM. The value obtained at the lower concentration was normalized by that obtained at the higher concentration, providing a more accurate concentration dependence. In the case of the plate-reader, different concentrations were observed in different wells (Fig. S6). In the case of the ATPase activities each set of fibers was first incubated in a lower concentration of piperine in a relaxing solution, followed by measurement of ATPase activities at that concentration and finally by measurement in 100 µM piperine (Fig. S7).
Fig. S6.
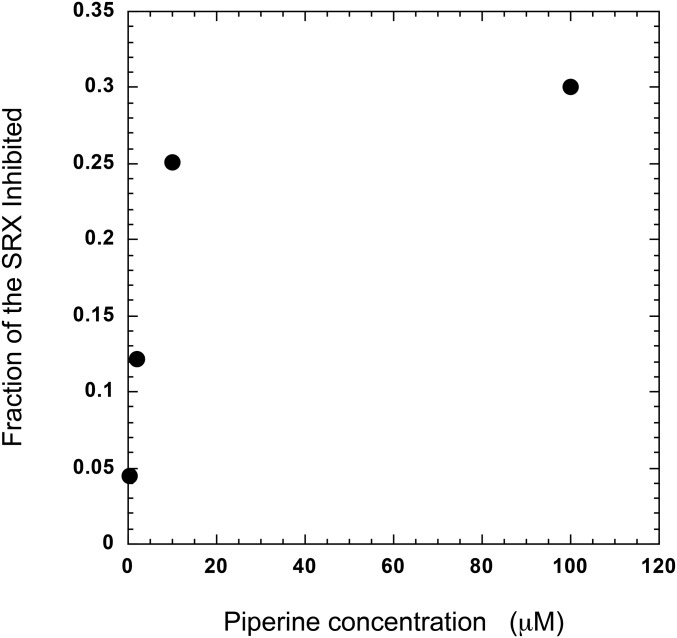
Measuring the Kd for the inhibition of the SRX in plates. Chopped, labeled fibers were pipetted into wells in a 384-well plate. The negative controls contained relaxing buffer, and positive controls contained rigor buffer. Experimental wells contained relaxing buffer plus different concentrations of piperine. As the piperine concentration increased it had a greater effect in decreasing the ratio of intensities, as shown for 100 µM piperine in Fig. 1. Results from the lower concentrations of piperine were then divided by that obtained at the highest concentration to produce a fractional inhibition. In this way the concentration dependence of different data sets can be compared more accurately. This normalization was needed to compare data sets because in different runs different maximal inhibitions were observed. In the one shown here the data appear to plateau close to 30% inhibition, whereas in other runs it reached a different plateau. The average plateau was 46%, as shown in Fig. 4.
Fig. S7.
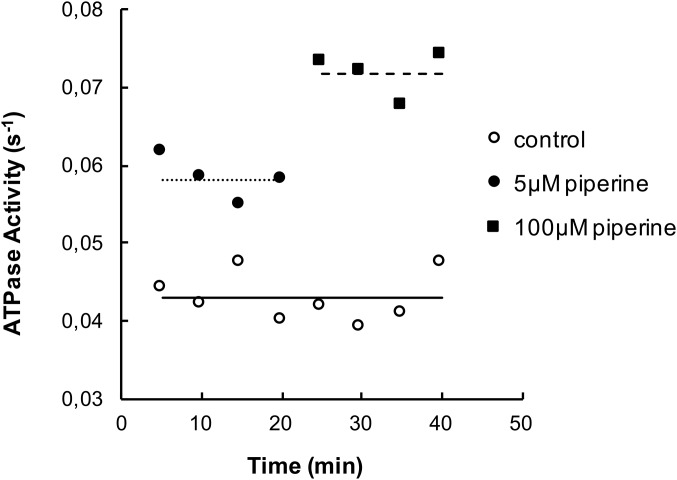
Measuring the ATPase activity of muscle samples. A bundle of muscle fibers, 10–20, was dissected and cut into two equal pieces. Fibers in each half were separated from each other to allow rapid diffusion and placed into two different vials. In the first vial they were incubated in 50 µL of relaxing buffer (open circles). In the second vial they were incubated in 50 µL relaxing buffer plus 5 µM piperine (solid circles). After 5 min of incubation the solution in each vial was removed, saved for phosphate determination, and replaced by a new solution. The incubations were repeated a total of eight times. After four assays the solution in the second vial was replaced by relaxing buffer plus 100 µM piperine (solid squares). The graph shows the ATPase activity for each incubation, normalized by the concentrations of myosin heads as described in Materials and Methods. Horizontal lines show the means of the different data sets: control, 0.044 s−1; 5 µM piperine, 0.058 s−1; 100 µM piperine, 0.072 s−1. As shown, the data for 5 µM piperine are intermediate between the control values and those for 100 µM piperine. To compare data sets the results in the lower concentration of piperine were normalized to those for 100 µM piperine, so that they could be expressed as a fraction of maximal activation. Results from a number of assays had to be averaged to produce the curve shown in Fig. 4B.
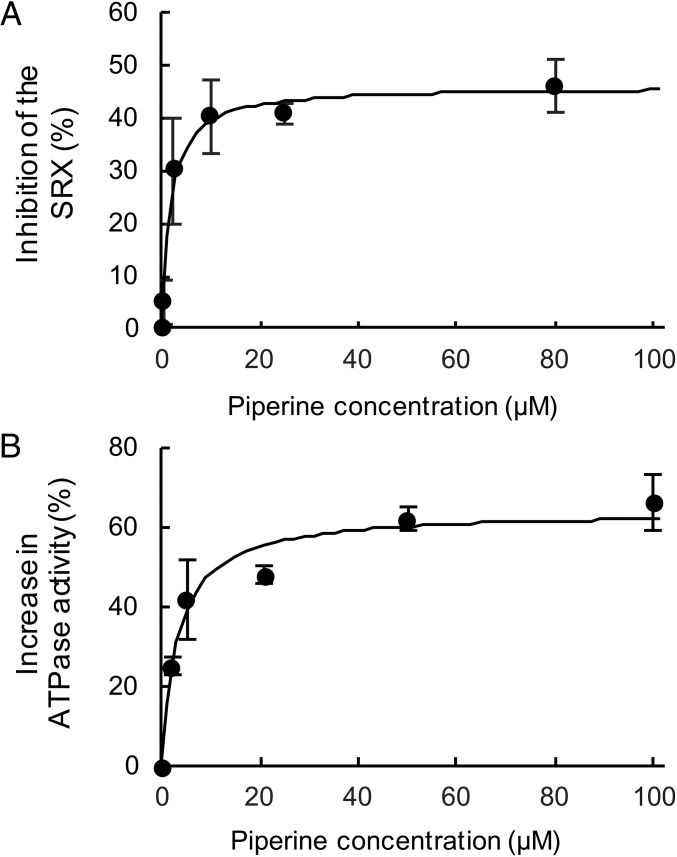
As the concentration of piperine was lowered, its effect on the ratio of fluorescent intensities and on the fiber ATPase activities decreased. Measurement of the fluorescent intensities in plates produced a Kd of 1.7 ± 0.4 μM (Fig. 4A). Observation of ATPase activities showed a similar Kd of 3 ± 0.8 μM (Fig. 4B). The affinity of piperine for its target is not sufficiently high to be used as a pharmaceutical in humans. However, it would be a good candidate as a lead compound for molecule optimization.
Fig. 4.
The concentration dependence of the effects of piperine on the SRX in fast skeletal fibers. (A) The inhibition of the SRX by piperine was measured using the ratio of fluorescent intensities of fibers in wells in a 384-well plate with different concentrations of piperine. Values are calculated by taking the rigor state as 0% SRX and the ATP state as 100% SRX. The fit to the data defines a Kd of 1.7 ± 0.4μM. (B) The effect of different concentrations of piperine on the increase of fiber ATPases, obtained as shown in Fig. 3. Values from different experiments were compared as described in Fig. S7. The fit to the data defines a Kd of 3 ± 0.8 μM. Errors on the data are SEM (n = 4–12).
Mechanics.
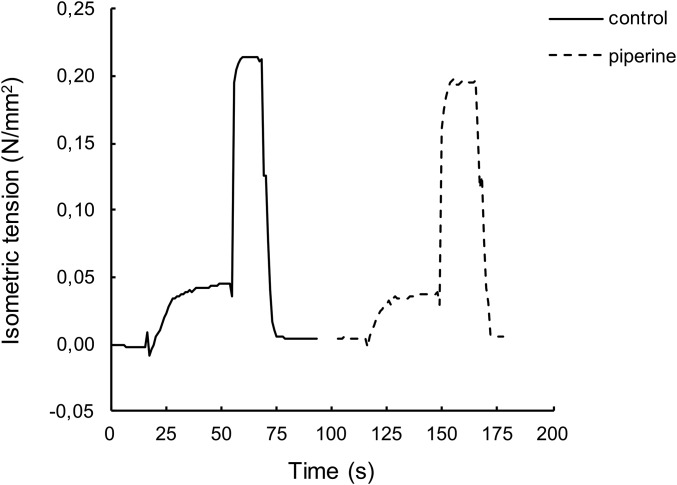
To be an effective therapeutic for metabolic diseases, a compound must increase the metabolic rate of resting fibers without having an effect on the mechanics of active muscle. To explore this possibility, single skinned muscle fibers were mounted on a tensiometer, which could measure tension and shortening velocities (28). Each fiber was observed in the presence or absence of 100 µM piperine, at 25 °C, using a temperature jump protocol to provide better sarcomere stability at 25 °C (Fig. S8). In fully relaxed fibers, piperine had no effect. Addition of 100 µM piperine to fully activated fibers had no significant effect on isometric tension, ratio of piperine to control = 1.05 ± 0.05 (SEM, n = 8) (Fig. S8). Isotonic velocities at 25% of isometric tension were measured (Fig. S9). Piperine had no significant effect on the shortening velocity, ratio of piperine to control = 0.97 ± 0.08 (SEM, n = 10).
Fig. S8.
Force is shown as a function of time during the temperature jump experiments as described in Materials and Methods. The fiber started in a relaxing solution. At 20 s it was transferred to an activating solution at 5 °C where it equilibrated with calcium. At 55 s it was transferred to an activating solution at 25 °C for a period of 10 s, where tension plateaued. At 70 s it was returned to the relaxing solution. Recording was paused at 92 s for several minutes. The activating solutions were changed to ones that contained 100 μM piperine, and the sequence was repeated. The ratio of isometric tensions in the data shown, +/− piperine, was 0.91. In a series of such experiments, the sequence of the solutions was alternated.
Fig. S9.
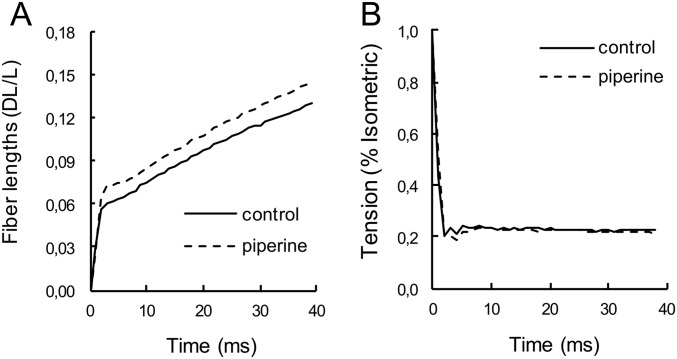
Measuring isotonic velocities during a load clamp. (A) The fractional change in fiber length and (B) the tension during the load clamp, in the absence (solid lines) and presence (dashed lines) of 100 μM piperine. A fiber was activated by the sequence of solutions and temperatures as shown in Fig. S8. At the peak of tension in the 25 °C bath, the fiber was switched to a load clamp to 22% of its isometric tension. The fiber shortened, first in an elastic response followed by a steady state. The tension drops dramatically followed by a period of stable tension. The data between 10 and 40 ms were fit by straight lines, whose slopes are the shortening velocities. The shortening velocity was 2.0 lengths · s‒1 in control solution and 2.2 lengths · s‒1 in piperine.
Components of Piperine.
Once a compound with the desired properties has been identified in a high-throughput screen, a customary next step is to examine similar compounds for further drug development. Piperine consists of two ring systems connected by a short conjugated leash (Fig. 1). The compound is easily broken down into two components: piperidine, the six-membered ring on the left, and piperic acid. The effects of both of these compounds were examined using the single-nucleotide turnover protocols. Neither compound at concentrations up to 200 µM had any effect on either the population or the lifetime of the SRX. Thus, the action of piperine requires that the two ring systems be connected together to produce an effect.
Discussion
After identifying piperine in the high-throughput screen as a destabilizer of the SRX, we were pleased to find in the literature that piperine had already been associated with attenuation of weight gain in rodents. Piperine is efficiently absorbed by the gut and is widely distributed in the various tissues (29, 30). However, most of the piperine has been conjugated with glucuronic acid, which may influence its activity. In a typical experiment, rats or mice were fed a high-fat chow for an extended time. All animals gained fat mass, but those also receiving piperine gained less than the controls. The difference in fat mass gain varied between 20 and 70% in different studies (31–35). Changes in lean mass were small, ∼10%. One study showed that administration of piperine to mice during caloric restriction had no effect on a series of parameters (36). Thus, the main effect of piperine appears to be to mitigate fat gain during caloric overload. In the studies above there was no change in the amount of food consumed; therefore piperine must affect the amount of fat stored by increasing the amount metabolized. Piperine is approved by the FDA for human consumption but at doses, 20 mg/d, much lower than used in the experiments in rodents cited above, 20–50 mg · kg‒1 · d‒1. It is approved, not for weight control, but for increasing the bioavailability of other drugs, which it does by inhibiting liver enzymes that metabolize them (37).
Piperine also has a beneficial effect in rodent models of type 2 diabetes (31, 32). Rodents fed high-fat and glucose diets for extended periods have higher blood glucose levels and show insulin resistance. Addition of piperine to their diets lowers levels of blood glucose and insulin. Piperine also improves the rate of glucose removal in a glucose tolerance test (32). All of the above observations could be explained by an increase in glucose consumption by resting muscle resulting from the increased metabolic rate of myosin produced by piperine.
The mechanisms by which piperine promotes weight loss or improves diabetes were unclear. A number of targets have been identified, but none have been shown to be causative (31, 33, 38–40). Our data show that piperine enhances thermogenesis of resting muscle via a perturbation of the SRX/DRX ratio, thus providing a mechanism. As originally recognized by Ferenczi and coworkers (14) over 30 y ago, in vivo ATP turnover requires almost all myosin in relaxed skeletal frog muscle to be in what we now term the SRX. A transfer of only 20% of the myosin in relaxed muscle from the SRX to the DRX would cause thermogenesis of resting muscle to double (5).
Could the activation of resting muscle metabolism by piperine found here explain the difference in fat gain produced by piperine in rodents fed high-fat chow for an extended period? In the experiments by BrahmaNaidu et al. (31), rats were fed high-fat chow for 42 d, producing one of the largest observed gains in fat mass. All rats gained fat during this time, but those who were also fed piperine gained ∼80 g less fat than controls. The oxidation of this amount of fat will yield 40 mol of ATP (41). We first compare this value to the amount of ATP that would be consumed if all of the myosin heads were in the DRX. The amount of myosin is calculated by taking the lean mass of the rats consuming the high-fat chow, 375 g, assuming 50% is muscle and multiplying by the concentration of myosin heads in mammalian muscle, 360 µM (42), to give 72 μmol of myosin. The basal ATPase activity of rat myosin has, to our knowledge, not been measured. To estimate this rate we extrapolated from the values for other myosin using the known variation of basal metabolic rate with body mass (43), producing an estimate of ∼0.5 s−1 (see Supporting Information for a more detailed description). Multiplying the total amount of myosin heads times the activity and the duration of the experiment we find that 129 mol of ATP would be consumed if all myosin heads were in the DRX. To estimate the fraction of the myosin heads that would be transferred from the SRX to the DRX, we assume that 50% of the heads are affected and only in fast twitch fibers, taken to be 50% of total fibers, giving an ATP consumption of ∼32 mol. This energy is similar to the energy involved in the difference in fat gain observed by BrahmaNaidu et al. (31). Thus, if the piperine concentration used was far above its Kd, >5 µM, it would explain much if not all of the attenuation in fat gain. BrahmaNaidu et al. measured the attenuation of fat gain at different concentrations of piperine, finding only a modest decrease in effect when the piperine dose was lowered from 40 mg · kg‒1 to 20 mg · kg‒1, suggesting that the doses used were far above the Kd (31). Although it requires a number of assumptions, this calculation suggests that the metabolic changes found here for piperine are of the magnitude to explain its effect on weight gain. The changes amount to an increase in the basic metabolic rate of about 25%. We propose that this is a major factor in the effect of piperine in mitigating weight gain and diabetes.
At saturating levels, piperine destabilizes ∼50% of the myosin heads that are in the SRX. This may be because the binding of piperine only provides enough energy to destabilize 50% of the heads in the array. Alternatively, the two myosin heads in the interacting-heads motif are in different configurations, and one of them, the free head, is less stable (15). It could be that piperine acts on that head only.
Although the affinity of piperine for its target is reasonably high, sufficient to produce beneficial effects at the high doses used in rodents, it is probably not high enough to be an effective therapy in humans. Piperine has been identified as binding to a number of other molecular targets with a similar affinity, discussed above, which would probably lead to unfavorable side effects if taken at the quantities that would be necessary for effective thermogenesis. Although piperine is probably not an effective therapeutic, it is an excellent lead compound, which could be used to find similar compounds whose properties could be optimized using our in vitro muscle assay systems.
Piperine has many of the qualities that would be required for any compound to be useful as a therapeutic treatment for metabolic diseases in humans: (i) It destabilizes the SRX and can lead to substantial thermogenesis. (ii) It functions only in fast twitch muscle fibers with a marginal effect in cardiac muscle fibers. (iii) It has little effect on fully active muscle fibers. (iv) It is well tolerated at high doses in rodents, with no obvious side effects. If a piperine-like pharmaceutical were developed that destabilized 50% of the myosin heads in the fast muscles of a 70-kg human [assuming 50% fast fibers and a myosin activity in the DRX of 0.09 s−1 (44)], it would increase metabolic rate by 1.2 MJ · d‒1. This represents 15% of total daily energy expenditure (TEE) and would consume 32 g fat · d‒1 or 12 kg fat · y‒1. How well will an increase of 15% of TEE be tolerated? Overexpression of uncoupling protein 3 or ectopic expression of uncoupling protein 1 in mice raised TEE by 15–25% (45–47). It was also well tolerated and led to decreased adiposity and improved insulin resistance, suggesting that the approach proposed here could be successful.
In summary, our results provide the proof of concept that pharmaceuticals targeting resting muscle thermogenesis can be found and that they will effectively treat the metabolic diseases, obesity and type 2 diabetes, in humans. Muscle is an ideal tissue to target for increasing thermogenesis, as it has a large-reserve metabolic capacity, and the modest increase suggested here can be accommodated. These pharmaceuticals will directly address the fundamental problem in these conditions: the consumption of more fuels than are metabolized. Here we show that high-throughput screens can be performed and that the protocols we used can find molecules that do increase the metabolic rate of resting muscle. Given the immensity of the problem and the need for effective therapies, this approach should be attempted. We suggest that this will open up an area in the field of muscle research and a race to be the first to market with a new class of pharmaceuticals.
Materials and Methods
Fibers and Solutions.
White adult New Zealand rabbits were killed according to protocols approved by the University of California, San Francisco Institutional Animal Care and Use Committee #AN108976-02. Psoas and soleus muscle fibers were harvested and stored at ‒20 °C in a solution of rigor buffer and glycerol mixed 50/50. The Rigor buffer contained 50 mM 3-(N-morpholino)propanesulfonic acid, 120 mM potassium acetate, 5 mM magnesium chloride, 5 mM EGTA, 4 mM DTT, 5 mM potassium phosphate, pH = 6.8. Relaxing buffers were obtained by addition of 4 mM ATP or 250 μM mantATP to the Rigor buffer. Activating solutions were obtained by addition of 3 mM CaCl2 to the relaxing buffer. Strips of rabbit cardiac tissues were obtained from the left ventricle, mounted with aluminum clips, and measured as described in ref. 48.
Proteins.
The RLC used was from mouse skeletal muscle (MLC2F, National Center for Biotechnology Information identification NP_058034.1) and was expressed in bacteria, labeled, and exchanged into fibers as described in ref. 18. Myosin was made as described by ref. 49. Labeling with MDCC was 60%, and ∼50% of the endogenous RLC was replaced by mutant RLC during the exchange (18).
Characterization of Fiber Properties.
Single-nucleotide turnovers were measured in flow cells as described previously (12). The ATPase activities of a group of single fibers, 8–12, were measured by direct determination of phosphate using malachite green (50), For a more detailed description of the assay, see Figs. S5 and S7. At concentrations of piperine of 25 µM and below the fibers were preincubated in piperine in relaxing solution for 60–120 min. Single-fiber mechanics were measured using methods and apparatus described previously (28) and in Figs. S8 and S9. All experiments were performed at room temperature, ∼22 °C. For more detailed descriptions, see Supporting Information.
High-Throughput Screening.
Fibers were chopped, producing a preparation of predominantly single fibers 50–200 µm in length (Fig. S1). The fibers were exchanged with the labeled light chains as described in Supporting Information and in ref. 18. The labeled fibers were loaded into the wells of 384-well plates. Fibers in relaxing solution without compounds served as negative controls, fibers in rigor buffer served as positive controls, and fibers in relaxing solution with 10-µM compounds were the experimental samples.
Images.
The images were collected using epifluorescence on a Nikon 6D High-Throughput fluorescent microscope. The excitation filter was 382–393 nm, and emission filters were set at 420–460 nm and 500–550 nm. Two images obtained at different emission wavelengths for each well were first analyzed by a macro written in Fiji (51). The macro used a series of cutoff filters to determine the intensities of the fibers; the background; and bright objects, such as dust, clumps of fibers, and so forth. After correction for background and bright spots, fiber fluorescence was determined and used to calculate the ratio of fiber intensity in the two images, providing a signal that was sensitive to the population of the SRX. Possible hits were checked by analyzing individual wells, via picking fibers and their adjacent background manually. The macro was able to analyze most wells, leaving only a few dozen wells with an outlying ratio in each plate to be manually checked.
Exchanging RLC on Muscle Fibers
Bundles of fibers were chopped with a scalpel blade on a cold glass slide and washed with exchange buffer for 1 h at 4 °C (Fig. S1). The resulting preparation consisted of predominately single fibers 50–200 µm in length. The chopped fibers were incubated with an equal volume of exchange buffer containing 100 µM of an RLC mutant with a single cysteine at position 5 (RLC-C5) labeled with MDCC. The mixture was again left shaking on ice for another hour and then put into a water bath at 30 °C for 30 min while gently stirring. The chopped fibers were washed in rigor solution containing 0.4 mg · mL‒1 solution of Troponin C and left shaking on ice for 1 h. In the end, the fibers were placed into a rigor solution with 50% glycerol, left shaking on ice for another 1–2 h, and stored at ‒20 °C for use. To load the fibers in the plate, a small volume of concentrated fibers was washed three times in cold rigor buffer plus 0.02% Triton X-100, centrifuged at 4,000 × g for 1 min, and resuspended in the same buffer. Each well contained ∼15 µg of total protein.
Brief Description of the High-Throughput Screening
Skinned chopped muscle fibers were exchanged with MDCC labeled RLC-C5 and used for the screen in 384-well plates, 24 columns of 16. All wells contained 60 µL of rigor buffer plus 0.02–0.05% Triton X-100. Columns 1–22 contained relaxing buffer. Column 1 had no fibers and was used to obtain pictures of the illuminated background. Chopped fibers were manually distributed in columns 2–24. Compounds, final concentration equal to 10 µM, were pinned in wells of columns 3–22. Wells in columns 23 and 24 had no ATP and were used as positive controls; column 2 was the negative control.
Compounds
The Small Molecule Discovery Center drug collection contains 2,177 Food and Drug Administration (FDA)- or European Union-approved clinical drugs, assembled from the commercially available Pharmakon 1600 collection (Micro Source Discovery Systems) and supplemented with the addition of approved small-molecule drugs sourced directly from vendors (Enzo Life Sciences; Tocris Bioscience) and smaller historical collections (Iconix FDA and bioactives set).
Images
The images were collected using epifluorescence on a Nikon 6D High-Throughput fluorescent microscope. The excitation filter was 382–393 nm, and emission filters were set at 420–460 nm and 500–550 nm. The shutter time used was 500 ms for each picture.
Fiji Macro
A Fiji (51) macro was developed to analyze the images. To obtain a ratio, the macro had to analyze two images of the same sample per well, one with the 420–460 nm emission and one with the 500–550 nm emission. The images were first corrected by subtracting an average background taken in empty wells, one for each channel. This procedure was added to make the excitation more uniform, especially at the corners, and to improve the reliability of the following steps. Dust and other objects on occasion resulted in bright spots that altered analysis. These were detected by a high cutoff filter and removed by setting those pixels to zero in both images. The image with the best contrast was used to autothreshold the fibers in the well. The fiber selection was used for both images, so that the measured area would be the same in each. To avoid scattering of light by fibers into the background region, the fiber selection was enlarged by 20 pixels before the inversion to select the background. The final output contained five values for each pair of images, fluorescence intensities for both channels, background intensities for both channels, and a correction coefficient. This last number represents the amount of pixels that had to be taken to zero because of the high brightness; by comparing this value with the size of a picture a reliability value can be estimated.
How to Clean Up Results
As mentioned in the previous section, some factors introduce noise into the data. Some of the molecules used in the screen were fluorescent in the range of one or both of the two emissions. In some wells there were too few fibers or clumps of fibers. All of these factors introduced noise into the data. The data were cleaned up by analyzing individual wells via picking fibers and adjacent background manually and by discarding data where autofluorescence was too intense. The macro was able to analyze most wells, leaving only a few dozen wells with an outlying ratio to be manually checked.
Measuring Fiber Mechanics
Single skinned muscle fibers were mounted on a tensiometer, which could measure tension and shortening velocities (28). The skinned fibers measured here are prone to progressive sarcomere heterogeneity, leading to lower forces and velocities, particularly at higher temperatures. This problem was diminished by first activating the fibers at a low temperature and then transferring them to a warmer temperature for a few (5–10) seconds as described previously (52). In a typical experiment, fibers were first activated at 5 °C for approximately 30 s to allow equilibration of calcium, nucleotides, and piperine, if added. The fiber was rapidly moved to an activating solution at 25 °C, and isometric tensions and/or shortening velocities were recorded before returning the fiber to the cooler solution. The solutions in both baths were then exchanged for ones that contained 100 µM piperine, and the experiment was repeated (Figs. S8 and S9). The order in which solutions were used was varied. To measure shortening velocities, after a few seconds in the warm bath, the tension was dropped to 25% of the isometric value, allowing the fiber to shorten over a period of 40 ms.
Estimating the Basal ATPase Activity of Rat Myosin
We were unable to find a value for the basal ATPase activity of rat myosin in the literature. To approximate it we extrapolated from sources where the myosin ATPase at physiological temperatures is known, using the well-known dependence of metabolic rate on body size. The basal metabolic rate (BMR) changes as mass2/3 (43). The mass-specific metabolic rate, BMR divided by mass, changes as mass‒1/3. We assume that myosin ATPase activity scales with mass-specific metabolic rate. The mass of the rabbits used in our laboratory was 4.6 kg. The mass of the control rats in the experiment under consideration was 360 g, smaller by a factor of 12.8. The basal metabolic rate of rabbit myosin was measured at physiological temperatures, 0.2 s−1, close to that found by ref. 53. Using the above formula, we calculate that the rate for rat myosin is 0.47 s−1. A similar calculation using data from humans, weight = 70 kg, myosin ATPase activity for fast muscle myosin at 37 °C = 0.09 s−1 (44), leads to a value for the rat myosin of 0.51 s−1. We estimate rat myosin ATPase activity at physiological temperatures as ∼0.5 s−1.
Acknowledgments
We thank Dr. Kurt Thorn and Ms. Delaine Larson for their generous help in using the microscopes. Troponin C used in the preparation of labeled fibers was a gift from Dr. Wen-ji Dong (Washington State University). The screen was run by Kenny Ang at the Small Molecule Discovery Center at University of California, San Francisco (UCSF), Michelle Arkin, director. We thank Fondazione Cassa di Risparmio di Padova e Rovigo for its support. This research was supported by a grant from the NIH (AR062279) (to E.P. and R.C.). Data for this study were acquired at the Nikon Imaging Center at UCSF/California Institute for Quantitative Biosciences (QB3).
Footnotes
Conflict of interest statement: R.C. is a member of the scientific advisory board of MyoKardia.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1607536113/-/DCSupplemental.
References
- 1.Ogden CL, Carroll MD, Fryar CD, Flegal KM. Prevalence of obesity among adults and youth: United States, 2011-2014. NCHS Data Brief. 2015;(219):1–8. [PubMed] [Google Scholar]
- 2.Gallagher J. 2016 Deadly diabetes in ‘unrelenting march.’ Health, BBC News Website. Available at www.bbc.com/news/health-35959554. Accessed April 6, 2016.
- 3.Levine JA, Eberhardt NL, Jensen MD. Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science. 1999;283(5399):212–214. doi: 10.1126/science.283.5399.212. [DOI] [PubMed] [Google Scholar]
- 4.Bouchard C, Blair SN, Katzmarzyk PT. Less sitting, more physical activity, or higher fitness? Mayo Clin Proc. 2015;90(11):1533–1540. doi: 10.1016/j.mayocp.2015.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Cooke R. The role of the myosin ATPase activity in adaptive thermogenesis by skeletal muscle. Biophys Rev. 2011;3(1):33–45. doi: 10.1007/s12551-011-0044-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Apovian CM, Garvey WT, Ryan DH. Challenging obesity: Patient, provider, and expert perspectives on the roles of available and emerging nonsurgical therapies. Obesity (Silver Spring) 2015;23(Suppl 2):S1–S26. doi: 10.1002/oby.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodgers RJ, Tschöp MH, Wilding JP. Anti-obesity drugs: Past, present and future. Dis Model Mech. 2012;5(5):621–626. doi: 10.1242/dmm.009621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Srinivasan S, Florez JC. Therapeutic challenges in diabetes prevention: We have not found the “exercise pill”. Clin Pharmacol Ther. 2015;98(2):162–169. doi: 10.1002/cpt.146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fothergill E, et al. Persistent metabolic adaptation 6 years after “The Biggest Loser” competition. Obesity. 2016;24(8):1612–1621. doi: 10.1002/oby.21538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Friedlander AL, Casazza GA, Horning MA, Usaj A, Brooks GA. Endurance training increases fatty acid turnover, but not fat oxidation, in young men. J Appl Physiol. 1999;86(6):2097–2105. doi: 10.1152/jappl.1999.86.6.2097. [DOI] [PubMed] [Google Scholar]
- 11.Ainsworth BE, et al. 2011 Compendium of Physical Activities: A second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 12.Stewart MA, Franks-Skiba K, Chen S, Cooke R. Myosin ATP turnover rate is a mechanism involved in thermogenesis in resting skeletal muscle fibers. Proc Natl Acad Sci USA. 2010;107(1):430–435. doi: 10.1073/pnas.0909468107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McNamara JW, Li A, dos Remedios CG, Cooke R. The role of super-relaxed myosin in skeletal and cardiac muscle. Biophys Rev. 2015;7:5–14. doi: 10.1007/s12551-014-0151-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ferenczi MA, Homsher E, Simmons RM, Trentham DR. Reaction mechanism of the magnesium ion-dependent adenosine triphosphatase of frog muscle myosin and subfragment 1. Biochem J. 1978;171(1):165–175. doi: 10.1042/bj1710165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alamo L, et al. Conserved intramolecular interactions maintain myosin interacting-heads motifs explaining tarantula muscle super-relaxed state structural basis. J Mol Biol. 2016;428(6):1142–1164. doi: 10.1016/j.jmb.2016.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Alamo L, et al. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol. 2008;384(4):780–797. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craig R, Woodhead JL. Structure and function of myosin filaments. Curr Opin Struct Biol. 2006;16(2):204–212. doi: 10.1016/j.sbi.2006.03.006. [DOI] [PubMed] [Google Scholar]
- 18.Nogara L, et al. Spectroscopic studies of the super relaxed state of skeletal muscle. PloS One. 2016;11(8):e0160100. doi: 10.1371/journal.pone.0160100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Xu S, Offer G, Gu J, White HD, Yu LC. Temperature and ligand dependence of conformation and helical order in myosin filaments. Biochemistry. 2003;42(2):390–401. doi: 10.1021/bi026085t. [DOI] [PubMed] [Google Scholar]
- 20.Aigner S, et al. Fast myosin heavy chain diversity in skeletal muscles of the rabbit: heavy chain IId, not IIb predominates. Eur J Biochem. 1993;211(1-2):367–372. doi: 10.1111/j.1432-1033.1993.tb19906.x. [DOI] [PubMed] [Google Scholar]
- 21.Swynghedauw B. Developmental and functional adaptation of contractile proteins in cardiac and skeletal muscles. Physiol Rev. 1986;66(3):710–771. doi: 10.1152/physrev.1986.66.3.710. [DOI] [PubMed] [Google Scholar]
- 22.Schiaffino S, Reggiani C. Molecular diversity of myofibrillar proteins: Gene regulation and functional significance. Physiol Rev. 1996;76(2):371–423. doi: 10.1152/physrev.1996.76.2.371. [DOI] [PubMed] [Google Scholar]
- 23.Candau R, Iorga B, Travers F, Barman T, Lionne C. At physiological temperatures the ATPase rates of shortening soleus and psoas myofibrils are similar. Biophys J. 2003;85(5):3132–3141. doi: 10.1016/S0006-3495(03)74731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman YE. Kinetics of the actomyosin ATPase in muscle fibers. Annu Rev Physiol. 1987;49:637–654. doi: 10.1146/annurev.ph.49.030187.003225. [DOI] [PubMed] [Google Scholar]
- 25.Szentesi P, Zaremba R, van Mechelen W, Stienen GJ. ATP utilization for calcium uptake and force production in different types of human skeletal muscle fibres. J Physiol. 2001;531(Pt 2):393–403. doi: 10.1111/j.1469-7793.2001.0393i.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Farman GP, et al. Blebbistatin: Use as inhibitor of muscle contraction. Pflugers Arch. 2008;455(6):995–1005. doi: 10.1007/s00424-007-0375-3. [DOI] [PubMed] [Google Scholar]
- 27.Straight AF, et al. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299(5613):1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- 28.Karatzaferi C, Franks-Skiba K, Cooke R. Inhibition of shortening velocity of skinned skeletal muscle fibers in conditions that mimic fatigue. Am J Physiol Regul Integr Comp Physiol. 2008;294(3):R948–R955. doi: 10.1152/ajpregu.00541.2007. [DOI] [PubMed] [Google Scholar]
- 29.Bhat BG, Chandrasekhara N. Metabolic disposition of piperine in the rat. Toxicology. 1987;44(1):99–106. doi: 10.1016/0300-483x(87)90049-7. [DOI] [PubMed] [Google Scholar]
- 30.Volak LP, et al. Effect of a herbal extract containing curcumin and piperine on midazolam, flurbiprofen and paracetamol (acetaminophen) pharmacokinetics in healthy volunteers. Br J Clin Pharmacol. 2013;75(2):450–462. doi: 10.1111/j.1365-2125.2012.04364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.BrahmaNaidu P, et al. Mitigating efficacy of piperine in the physiological derangements of high fat diet induced obesity in Sprague Dawley rats. Chem Biol Interact. 2014;221:42–51. doi: 10.1016/j.cbi.2014.07.008. [DOI] [PubMed] [Google Scholar]
- 32.Choi S, et al. Piperine reverses high fat diet-induced hepatic steatosis and insulin resistance in mice. Food Chem. 2013;141(4):3627–3635. doi: 10.1016/j.foodchem.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 33.Kim KJ, Lee MS, Jo K, Hwang JK. Piperidine alkaloids from Piper retrofractum Vahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase. Biochem Biophys Res Commun. 2011;411(1):219–225. doi: 10.1016/j.bbrc.2011.06.153. [DOI] [PubMed] [Google Scholar]
- 34.Okumura Y, Narukawa M, Watanabe T. Adiposity suppression effect in mice due to black pepper and its main pungent component, piperine. Biosci Biotechnol Biochem. 2010;74(8):1545–1549. doi: 10.1271/bbb.100117. [DOI] [PubMed] [Google Scholar]
- 35.Prakash UN, Srinivasan K. Fat digestion and absorption in spice-pretreated rats. J Sci Food Agric. 2012;92(3):503–510. doi: 10.1002/jsfa.4597. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, et al. Caloric restriction favorably impacts metabolic and immune/inflammatory profiles in obese mice but curcumin/piperine consumption adds no further benefit. Nutr Metab (Lond) 2013;10(1):29. doi: 10.1186/1743-7075-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Atal CK, Dubey RK, Singh J. Biochemical basis of enhanced drug bioavailability by piperine: evidence that piperine is a potent inhibitor of drug metabolism. J Pharmacol Exp Ther. 1985;232(1):258–262. [PubMed] [Google Scholar]
- 38.Shah SS, et al. Effect of piperine in the regulation of obesity-induced dyslipidemia in high-fat diet rats. Indian J Pharmacol. 2011;43(3):296–299. doi: 10.4103/0253-7613.81516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diwan V, Poudyal H, Brown L. Piperine attenuates cardiovascular, liver and metabolic changes in high carbohydrate, high fat-fed rats. Cell Biochem Biophys. 2013;67(2):297–304. doi: 10.1007/s12013-011-9306-1. [DOI] [PubMed] [Google Scholar]
- 40.Park UH, et al. Piperine, a component of black pepper, inhibits adipogenesis by antagonizing PPARγ activity in 3T3-L1 cells. J Agric Food Chem. 2012;60(15):3853–3860. doi: 10.1021/jf204514a. [DOI] [PubMed] [Google Scholar]
- 41.Stryer L. Biochemistry. Freeman; New York: 1995. [Google Scholar]
- 42.Borina E, Pellegrino MA, D’Antona G, Bottinelli R. Myosin and actin content of human skeletal muscle fibers following 35 days bed rest. Scand J Med Sci Sports. 2010;20(1):65–73. doi: 10.1111/j.1600-0838.2009.01029.x. [DOI] [PubMed] [Google Scholar]
- 43.White CR, Seymour RS. Mammalian basal metabolic rate is proportional to body mass2/3. Proc Natl Acad Sci USA. 2003;100(7):4046–4049. doi: 10.1073/pnas.0436428100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Resnicow DI, Deacon JC, Warrick HM, Spudich JA, Leinwand LA. Functional diversity among a family of human skeletal muscle myosin motors. Proc Natl Acad Sci USA. 2010;107(3):1053–1058. doi: 10.1073/pnas.0913527107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Klaus S, Rudolph B, Dohrmann C, Wehr R. Expression of uncoupling protein 1 in skeletal muscle decreases muscle energy efficiency and affects thermoregulation and substrate oxidation. Physiol Genomics. 2005;21(2):193–200. doi: 10.1152/physiolgenomics.00299.2004. [DOI] [PubMed] [Google Scholar]
- 46.Son C, et al. Reduction of diet-induced obesity in transgenic mice overexpressing uncoupling protein 3 in skeletal muscle. Diabetologia. 2004;47(1):47–54. doi: 10.1007/s00125-003-1272-8. [DOI] [PubMed] [Google Scholar]
- 47.Li B, et al. Skeletal muscle respiratory uncoupling prevents diet-induced obesity and insulin resistance in mice. Nat Med. 2000;6(10):1115–1120. doi: 10.1038/80450. [DOI] [PubMed] [Google Scholar]
- 48.Hooijman P, Stewart MA, Cooke R. A new state of cardiac myosin with very slow ATP turnover: A potential cardioprotective mechanism in the heart. Biophys J. 2011;100(8):1969–1976. doi: 10.1016/j.bpj.2011.02.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Crooks R, Cooke R. Tension generation by threads of contractile proteins. J Gen Physiol. 1977;69(1):37–55. doi: 10.1085/jgp.69.1.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lanzetta PA, Alvarez LJ, Reinach PS, Candia OA. An improved assay for nanomole amounts of inorganic phosphate. Anal Biochem. 1979;100(1):95–97. doi: 10.1016/0003-2697(79)90115-5. [DOI] [PubMed] [Google Scholar]
- 51.Schindelin J, et al. Fiji: An open-source platform for biological-image analysis. Nat Methods. 2012;9(7):676–682. doi: 10.1038/nmeth.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pate E, Bhimani M, Franks-Skiba K, Cooke R. Reduced effect of pH on skinned rabbit psoas muscle mechanics at high temperatures: Implications for fatigue. J Physiol. 1995;486(Pt 3):689–694. doi: 10.1113/jphysiol.1995.sp020844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Myburgh KH, Franks-Skiba K, Cooke R. Nucleotide turnover rate measured in fully relaxed rabbit skeletal muscle myofibrils. J Gen Physiol. 1995;106(5):957–973. doi: 10.1085/jgp.106.5.957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cooke R, Franks K. All myosin heads form bonds with actin in rigor rabbit skeletal muscle. Biochemistry. 1980;19(10):2265–2269. doi: 10.1021/bi00551a042. [DOI] [PubMed] [Google Scholar]