Figure 5. Reciprocal control of Runx2 and PPARγ transcriptional activity by DDR2-dependent phosphorylation.
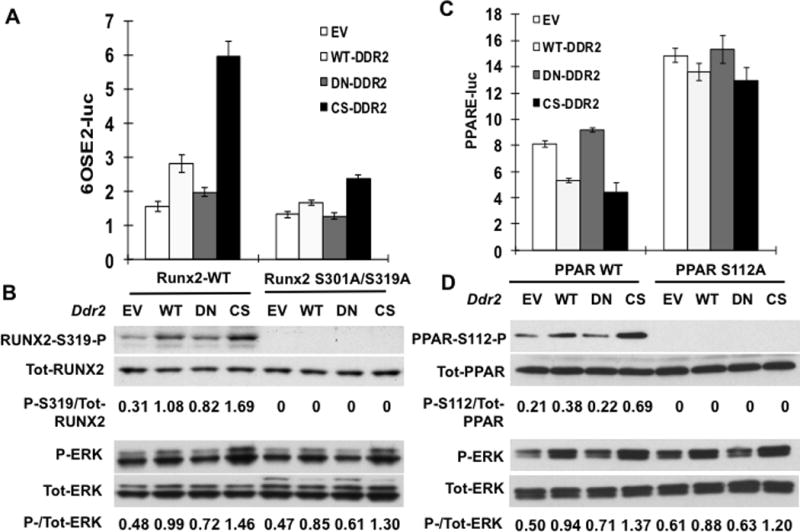
(A,B) Runx2 regulation. COS7 cells were transfected with a RUNX2 reporter plasmid (6OSE2-luc) and expression vectors encoding wild type (Runx2-WT) or phosphorylation site mutant RUNX2 (Runx2 S301A/S319A) and empty vector (EV), wild type (WT) or constitutively-active (CS) DDR2. (A) Normalized luciferase activity. (B) Immunoblot of RUNX2-S319-P, total RUNX2, P-ERK and total ERK. Values under each lane indicate ratios or P-RUNX2/total RUNX2 and P-ERK/total ERK as measured by densitometry. (C,D) PPARɣ regulation. Cells were transfected with PPARγ reporter/RXR combination (PPRE-luc) as described in Methods together with expression vectors encoding wild type (PPAR-WT) or phosphorylation site mutant PPARγ (PPAR-S112AA) and empty vector (EV), wild type (WT) or constitutively-active (CS) DDR2. (C) Normalized luciferase activity. (D) Immunoblot of PPARγ-S112-P, total PPARγ, P-ERK and total ERK.
