Abstract
Bones are an important component of vertebrates; they grow explosively in early life and maintain their strength throughout life. Bones also possess amazing capabilities to repair — the bone is like new without a scar after complete repair. In recent years, a substantial progress has been made in our understanding on mammalian bone stem cells. Mouse genetic models are powerful tools to understand the cell lineage, giving us better insights into stem cells that regulate bone growth, maintenance and repair. Recent findings about these stem cells raise new questions that require further investigations.
Introduction
One of the distinguishing features of vertebrates that explicitly discriminate them from invertebrates is their bones. Bones, with their strong and rigid structures due to mineralized matrix, provide protection for vital organs and act as levers whereby muscle contraction leads to movement of the body. Bones assume very different shapes in different part of the body, but they are formed through only two common mechanisms; intramembra-nous and endochondral bone formation. Intramembranous bone formation is a simple and straightforward process in which undifferentiated mesenchymal cells directly become osteoblasts that lay down the mineralized matrix. Intramembranous bones (or dermal bones) evolved earlier in the early fish, and comprise part of the skull in mammals. By contrast, endochondral bone formation is a complex process in which initial cartilage templates are replaced by bone. Most bones in mammals are formed through endochondral bone formation.
Because of their primary function, bones are among the most commonly injured tissues of the body. Despite their inert appearance, bones continually turn over, replacing old bone with new and possess amazing capabilities to repair even after bone growth slows or stops. Not surprisingly, bone repair recapitulates the developmental sequence of the two modes of bone formation. Characterizing stem cells for bone growth, maintenance and repair has been largely hampered until recently due to technical and conceptual difficulties, including handling of mineralized hard tissues, complexity and plasticity of the bone cell development and lack of stage-specific markers or active promoters/enhancers identified in the early bone cells. Over the past few years, we have seen a substantial increase in our knowledge on this field. In this mini-review, we will discuss recent advances in the study of mammalian bone stem cells.
Stem cells for bone growth
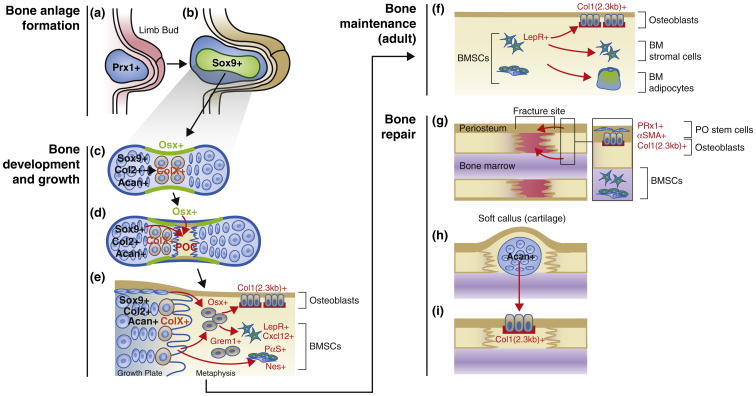
Bone growth is substantial in early life, gradually slows down and eventually stops in adulthood. Therefore, stem cells for bone growth play active roles in early life while gradually slowing their action in later life, although the homeostatic turnover of adult bone continues throughout life. The relationship between stem cells needed for bone growth and stem cells needed for continuing renewal of bone during bone remodeling is uncertain. We will discuss endochondral bone formation of the limb as an example. The limb originates from the lateral plate mesoderm during embryogenesis. The transcription factor Prrx1 is expressed in these mesodermal cells (Figure 1a). In fact, Prrx1-cre, in which cre recombinase is expressed under the direction of a 2.4 kb Prrx1 promoter, marks essentially all limb mesenchymal cells in bones at a later stage, including osteoblasts, chondrocytes and stromal cells, but not muscle satellite cells [1]. Subsequently, a group of early mesenchymal cells within the limb bud condenses and determines the domain for the future cartilage and bone. The transcription factor Sox9 is expressed in these mesenchymal cells (Figure 1b), and indeed is required for condensation [2]. These early mesenchymal cells develop into other mesenchymal cells in the cartilage and bone at a later stage, as Sox9-cre marks essentially all chondrocytes and osteoblasts, even though it is expressed only in mesenchymal precursors and in chondrocytes [3]. These fate-mapping experiments are consistent with the idea that stem cells for bone growth arise locally within the bone anlage. Sox9 directly binds to regulatory elements of cartilage-matrix genes, including those encoding type II collagen (Col2) and aggrecan (Acan). Cells within condensations start to express Col2 and differentiate into chondrocytes, which then differentiate into hypertrophic chondrocytes expressing type X collagen (ColX) (Figure 1c). Around the same time that chondrocyte hypertrophy occurs, osteoblast precursors first appear in the surrounding region termed the perichondrium (Figure 1c). These perichondrial cells invade into the cartilage template along with blood vessels and become osteoblasts and stromal cells, establishing the primary ossification center (Figure 1d). Runx2 and osterix (Osx), transcription factors essential for osteoblast differentiation [4–6], are expressed in a portion of the perichondrium near hypertrophic chondrocytes.
Figure 1.
Stem cells for bone growth, maintenance and repair. (a) Limb bud formation. Prrx1+ cells in the lateral plate mesoderm are the precursors for all other mesenchymal cells in bones at a later stage. (b) Mesenchymal condensation. Sox9+ cells are the precursors for all other chondrocytes and osteoblasts therefore determine the domain for the future bones. (c) Cartilage formation. Condensing mesenchymal cells soon differentiate into chondrocytes (Sox9+, Col2+ and Acan+) and establish the growth cartilage. These cells proliferate and further differentiate into hypertrophic chondrocytes (ColX+) at the center of the mold. The osteogenic perichondrium forms in its vicinity, where the first osteoblasts precursors (Osx+) appear. (d) Ossification. Blood vessels invade into the cartilage template attracted by angiogenic factors secreted by hypertrophic chondrocytes. Primary ossification center (POC) is formed when perichondrial and other mesenchymal cells from the cartilage move inside with blood vessels and proliferate. (e) Growth. The growth plate provides the primary engine for bone growth, and multiple types of mesenchymal cells are enlisted to form bones. A subset of growth plate cells (Sox9+, Col2+ and Acan+) continues to provide bone cells, some of which may pass through a ColX+ stage. Cells in the metaphysis, including Osx+ and/or Grem1+ cells are the precursors for osteoblasts and bone marrow stromal cells including BMSCs (LepR+, Cxcl12+, PαS+ and Nes+). (f) Maintenance. After bone growth stops, BMSCs (LepR+ and others) become the main source of osteoblasts, bone marrow stromal cells and adipocytes. (g) Fracture repair. When a complete fracture occurs, two distinct stem cell populations from the periosteum and the bone marrow respond to injuries. Periosteal stem cells (Prrx1+ and/or aSMA+) are the major source of chondrocytes in the soft callus, although BMSCs can contribute to chondrocytes to some extent. (H) Soft callus. Chondrocytes in the soft callus (Acan+) are the source of osteoblasts in the repaired bone. (I) Ossification of the fracture callus.
Lineage-tracing experiments using an Osx-creERt line demonstrate that fetal perichondrial Osx+ osteoblast precursors can indeed translocate into the ossification center [7]. As the bone anlage grows bigger, osteoblasts and stromal cells continue to proliferate as the primary ossification center expands. The perichondrial precursors that moved into the template can continue to proliferate only for a limited period, and eventually disappear [8•,9]. Interestingly, Osx+ cells marked in the early postnatal period, when the bone marrow is established, continue to generate stromal cells in the marrow for at least many months [8•,9]. Osx+ cells marked in adults do not have such capability [8•].
While the marrow space is being formed, the secondary ossification center develops within the epiphyseal cartilages remaining on both ends of the bone. The cartilage between the primary and secondary ossification centers is termed the growth plate, as it forms a disk with characteristics columns of chondrocytes (Figure 1e). The growth plate is the main engine for postnatal bone growth. Slowly proliferating resting or reserve chondrocytes sit atop of the growth plate, and probably serve as precursors for other columnar chondrocytes [10]. Recent studies suggest that chondrocytes or their close relatives within the growth plate or its surrounding areas serve as a major source of other bone lineage cells in growing bones (Figure 1e). Our recent study demonstrates that, using lineage-tracing experiments with multiple transgenes active in mesenchymal condensations and early growth cartilage (Col2-creERt, Sox9-creERt and Acan-creERt), a subset of early postnatal progenitor cells continues to provide osteoblasts, bone marrow stromal cells and adipocytes for over a year in mice [11••]. In addition, Col2-cre marks a majority of osteoblasts, stromal cells expressing a Cxcl12-GFP (‘CAR cells’), and bone marrow mesenchymal progenitors (CFU-Fs and PaS cells) (we will discuss these cells later). A lineage-tracing study using ColX-cre and Acan-creERt from another group demonstrates that chondrocytes may be the major source of osteoblasts in endochondral bones [12••]. Yet another group showed using ColX-cre and ColX-creERt that hypertrophic chondrocytes can become osteoblasts [13]. Therefore, these studies collectively suggest that chondrocytes themselves may also be a source of osteoblasts and stromal cells during endochondral bone formation, emphasizing that the possibility that multiple cell types are enlisted to form bone during rapid bone growth. Supporting this hypothesis, one group showed that ‘mouse skeletal stem cells (mSSCs)’, identified by a combination of cell surface markers and found in proximity to the growth plate, are capable of generating bone, cartilage, and stroma after transplantation under the kidney capsule [14]. Further, another group showed that, using Grem1-creERt (Gremlin 1 is an antagonist for bone morphogenic protein, BMP), osteochondroreticular (OCR) stem cells are concentrated within the metaphysis of long bones, but not in the perisinusoidal space [15]; these ‘OCR stem cells’ are more clonogenic than perisinusoidal bone marrow stromal cells marked with Nestin-GFP. While Grem1-creERt marks early cells that become chondrocytes and osteoblasts in vivo, the transgene does not mark adipocytes, illustrating further the variable fates of cells capable of generating multiple mesenchymal lineages. These recent studies support the notion that distinct types of stem cells for bone growth exist to support explosive growth of bone in early life.
Stem cells for bone maintenance
Even after bone growth slows or stops, bones need constant maintenance to sustain their structures and functions throughout the lifespan. Stem cells in adult bones play roles in the much slower remodeling process, and are hypothesized to reside mainly in bone marrow (Figure 1f). Marrow cells capable of forming colonies when plated on plastic, colony-forming unit fibroblasts (CFU-Fs), have been hypothesized to serve as stem cells of the mesenchymal cell lineage, also termed bone marrow stromal/stem cells (BMSCs) [16]. Many studies of these cells have used in vitro/ex vivo cell culture systems and heterotopic transplantation as a way to demonstrate precursor-product relationship. PDGFRα+Sca1+ nonhematopoietic cells (‘PαS cells’) reside in the perivascular space in vivo and are highly enriched for CFU-Fs [17]. Further, more importantly, co-transplantation of uncultured GFP+ PαS cells with hematopoietic stem cells (HSCs) into irradiated donor resulted in engraftment of GFP+ cells in bone marrow: they became osteoblasts, adipocytes, stromal cells, and PαS cells themselves, suggesting their self-renewal in vivo. Interestingly, cultured PαS cells did not have such capability. Nestin-GFP is expressed in perivascular stromal cells and pericytes of bone marrow arterioles (Nestin is an intermediate filament protein and a marker for neural stem cells) [18]. These Nes-GFP+ stromal cells include all CFU-F activities, and formed self-renewable ‘mesenspheres’ that could pass serial heterotopic transplantations. Cxcl12 (chemokine (C-X-C motif) ligand 12) is a stromal cell derived factor important for hematopoiesis, and Cxcl12-GFP+ stromal cells are perivascular reticular cells adjacent to sinusoids or in the endosteum, termed CAR (Cxcl12-abundant reticular) cells [19]. These cells express osteogenic transcription factors, Runx2 and Osx, and the adipogenic transcription factor, PPARγ, and behave as osteo-adipogenic progenitors in vitro. These studies cannot, however, address the function of stem cells in their native setting. A recent study reports that, using a leptin receptor (LepR)-cre knock-in allele, the descendants of LepR-cre-expressing cells contribute to a large fraction of CFU-Fs, and adult osteoblasts and adipocytes; in addition, these cells are intrafemorally transplantable after sublethal irradiation, indicating that perisinusoidal mesenchymal stromal cells expressing LepR are the main source of bone-forming cells in adult mice [20••]. Interestingly, LepR+ cells become osteoblasts only after two months of age. Because expression of LepR-cre cannot be limited to a particular time, the authors are limited in their ability to identify in vivo the specific precursors of adult osteoblasts and adipocytes. An inducible genetic model specific for BMSCs will be required to define more conclusively how these cells contribute to bone maintenance in vivo in adult bones. The first such endeavor made by Mendez-Ferrer et al. [18], which used a Nestin-creERt transgenic line, is exciting, but interpretation of their findings can be potentially confounded by heterogeneity of the cell types in which Nestin-creERt is expressed in bone. In particular, as Nestin-creERt trans-gene simultaneously marks endothelial cells at least during bone growth, we cannot exclude endothelial contribution [9]. Moreover, a study reports that different Nestin transgenes seem to be expressed by different subpopulations of perivascular stromal cells [21]. New genetic tools needed to define adult stem cells needed for bone homeostasis.
Stem cells for bone repair
Damage to bone structure can vary in severity from microscopic microfractures to fractures that completely disrupt bone continuity. Most of small and mechanically stable fractures heal by intramembranous bone formation, whereas large and unstable fractures also involve generation of fibrocartilages and soft callus at the center of the fracture site that undergo endochondral bone formation [22]. Bone repair requires the mobilization of stem cells to allow deposition of mineralized matrix at the injury site. Two major sources for these stem cells are the periosteum and the bone marrow (Figure 1g), although the other sources are also possible [23]. Two distinct types of stem cells, that is, periosteal stem cells and BMSCs differentially participate in bone repair; cells from the periosteum are the source of chondrocytes and osteoblasts, whereas cells from the bone marrow undergo osteoblast differentiation [24•]. Lineage-tracing experiments using a unicortical transplantation model and subsequent fracture experiments indicate that periosteal stem cells are the major source of chondrocytes, whereas BMSCs also contribute to chondrocytes but to a much lesser extent [24•]. In addition, in a special circumstance of neonatal angulated fractures, a bidirectional growth plate similar to the synchondroses of the cranial base forms at the concave side of the fracture to realign the fragments [25••]. Therefore, periosteal stem cells are likely to retain the capability to regenerate a growth plate after fracture, at least in early life. Interestingly, Prrx1 expression becomes confined to the periosteum in the cambium layer during a postnatal period, immediately outside the osteoblasts lining the bone surface [26]. A lineage-tracing study using Prrx1-creERt reveals that these periosteal cells give rise to some of the chondrocytes and osteoblasts in the fracture callus [27•], suggesting the possibility that Prrx1-expressing cells in the periosteum include stem cells retaining characteristics similar to those of limb bud mesenchymal cells during early morphogenesis. Another effective marker for periosteal cells is alpha-smooth muscle actin (αSMA). αSMA-creERt-marked periosteal cells expand in response to fracture and start to differentiate into chon-drocytes and osteoblasts [28••]. These lineage-tracing studies support the idea that a subset of periosteal cells is the principal source of chondrocytes and osteoblasts during fracture repair. The contribution of BMSCs to fracture repair has also been investigated. Bone marrow stromal cells derived from perinatal Osx-creERt+ cells [8•] or LepR-cre+ cells [8•,20••] contribute to chondrocytes in the fracture callus. However, because descendants of both of these cre-marked populations also include periosteal cells, these studies cannot conclusively exclude periosteal involvement. As in bone growth, chondrocytes in the soft callus may well become the source of osteoblasts in subsequent ossification (Figure 1h and i). A study shows that Acan-creERt marks chondrocytes in the fracture callus, and they subsequently become Col1(2.3 kb)-GFP+ osteoblasts in the fracture site [12••]. Despite these recent significant advances, further detailed analyses need to be undertaken to delineate the characteristics of stem cells for bone repair. Use of additional transgenic markers for molecular characterization of the cells and their heterogeneity will be essential to understand the contribution of each stem cell population to fracture repair. The fundamental properties of periosteal stem cells and how they differ from BMSCs need clarification.
Conclusion
Bones repair themselves marvelously, appearing to recapitulate, with variations, the process of development. Recent studies clearly suggest that stem cells for bone growth and maintenance are also relevant to bone repair. Our knowledge on these stem cells still lags behind understanding of development of many other organs in mammals. We need to better understand the heterogeneity of these cells, their molecular characterization, and their regulation. Particularly, we will need to develop more inducible genetic tools that can specifically mark potential bone stem cells for further functional analysis.
Acknowledgments
These authors gratefully acknowledge the support from National Institutes of Health Grants DE022564 to N.O. and DK011794 to H.M.K.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Logan M, Martin JF, Nagy A, Lobe C, Olson EN, Tabin CJ. Expression of Cre Recombinase in the developing mouse limb bud driven by a Prxl enhancer. Genesis. 2002;33:77–80. doi: 10.1002/gene.10092. [DOI] [PubMed] [Google Scholar]
- 2.Bi W, Deng JM, Zhang Z, Behringer RR, de Crombrugghe B. Sox9 is required for cartilage formation. Nat Genet. 1999;22:85–89. doi: 10.1038/8792. [DOI] [PubMed] [Google Scholar]
- 3.Akiyama H, Kim JE, Nakashima K, Balmes G, Iwai N, Deng JM, Zhang Z, Martin JF, Behringer RR, Nakamura T, de Crombrugghe B. Osteo-chondroprogenitor cells are derived from Sox9 expressing precursors. Proc Natl Acad Sci U S A. 2005;102:14665–14670. doi: 10.1073/pnas.0504750102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otto F, Thornell AP, Crompton T, Denzel A, Gilmour KC, Rosewell IR, Stamp GW, Beddington RS, Mundlos S, Olsen BR, Selby PB, Owen MJ. Cbfa1, a candidate gene for cleidocranial dysplasia syndrome, is essential for osteoblast differentiation and bone development. Cell. 1997;89:765–771. doi: 10.1016/s0092-8674(00)80259-7. [DOI] [PubMed] [Google Scholar]
- 5.Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;89:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- 6.Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B. The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 7.Maes C, Kobayashi T, Selig MK, Torrekens S, Roth SI, Mackem S, Carmeliet G, Kronenberg HM. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev Cell. 2010;19:329–344. doi: 10.1016/j.devcel.2010.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8•.Mizoguchi T, Pinho S, Ahmed J, Kunisaki Y, Hanoun M, Mendelson A, Ono N, Kronenberg HM, Frenette PS. Osterix marks distinct waves of primitive and definitive stromal progenitors during bone marrow development. Dev Cell. 2014;29:340–349. doi: 10.1016/j.devcel.2014.03.013. They demonstrate that Osx+ cells at an early postnatal period are the origin of bone marrow stromal/stem cells in adult bones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ono N, Ono W, Mizoguchi T, Nagasawa T, Frenette PS, Kronenberg HM. Vasculature-associated cells expressing nestin in developing bones encompass early cells in the osteoblast and endothelial lineage. Dev Cell. 2014;29:330–339. doi: 10.1016/j.devcel.2014.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abad V, Meyers JL, Weise M, Gafni RI, Barnes KM, Nilsson O, Bacher JD, Baron J. The role of the resting zone in growth plate chondrogenesis. Endocrinology. 2002;143:1851–1857. doi: 10.1210/endo.143.5.8776. [DOI] [PubMed] [Google Scholar]
- 11••.Ono N, Ono W, Nagasawa T, Kronenberg HM. A subset of chondrogenic cells provides early mesenchymal progenitors in growing bones. Nat Cell Biol. 2014;16 doi: 10.1038/ncb3067. We show that a subset of chondrogenic cells is a significant source of osteoblasts and bone marrow stromal cells in growing bones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12••.Zhou X, von der Mark K, Henry S, Norton W, Adams H, de Crombrugghe B. Chondrocytes transdifferentiate into osteoblasts in endochondral bone during development, postnatal growth and fracture healing in mice. PLoS Genet. 2014;10:e1004820. doi: 10.1371/journal.pgen.1004820. They demonstrate that chondrocytes transdifferentiate into osteoblasts during bone growth and fracture repair. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yang L, Tsang KY, Tang HC, Chan D, Cheah KS. Hypertrophic chondrocytes can become osteoblasts and osteocytes in endochondral bone formation. Proc Natl Acad Sci U S A. 2014;111:12097–12102. doi: 10.1073/pnas.1302703111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chan CK, Seo EY, Chen JY, Lo D, McArdle A, Sinha R, Tevlin R, Seita J, Vincent-Tompkins J, Wearda T, Lu WJ, Senarath-Yapa K, Chung MT, Marecic O, Tran M, Yan KS, Upton R, Walmsley GG, Lee AS, Sahoo D, Kuo CJ, Weissman IL, Longaker MT. Identification and specification of the mouse skeletal stem cell. Cell. 2015;160:285–298. doi: 10.1016/j.cell.2014.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Worthley DL, Churchill M, Compton JT, Tailor Y, Rao M, Si Y, Levin D, Schwartz MG, Uygur A, Hayakawa Y, Gross S, Renz BW, Setlik W, Martinez AN, Chen X, Nizami S, Lee HG, Kang HP, Caldwell JM, Asfaha S, Westphalen CB, Graham T, Jin G, Nagar K, Wang H, Kheirbek MA, Kolhe A, Carpenter J, Glaire M, Nair A, Renders S, Manieri N, Muthupalani S, Fox JG, Reichert M, Giraud AS, Schwabe RF, Pradere JP, Walton K, Prakash A, Gumucio D, Rustgi AK, Stappenbeck TS, Friedman RA, Gershon MD, Sims P, Grikscheit T, Lee FY, Karsenty G, Mukherjee S, Wang TC. Gremlin 1 identifies a skeletal stem cell with bone, cartilage, and reticular stromal potential. Cell. 2015;160:269–284. doi: 10.1016/j.cell.2014.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bianco P. Mesenchymal stem cells. Annu Rev Cell Dev Biol. 2014;30:677–704. doi: 10.1146/annurev-cellbio-100913-013132. [DOI] [PubMed] [Google Scholar]
- 17.Morikawa S, Mabuchi Y, Kubota Y, Nagai Y, Niibe K, Hiratsu E, Suzuki S, Miyauchi-Hara C, Nagoshi N, Sunabori T, Shimmura S, Miyawaki A, Nakagawa T, Suda T, Okano H, Matsuzaki Y. Prospective identification, isolation, and systemic transplantation of multipotent mesenchymal stem cells in murine bone marrow. J Exp Med. 2009;206:2483–2496. doi: 10.1084/jem.20091046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma'ayan A, Enikolopov GN, Frenette PS. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Omatsu Y, Sugiyama T, Kohara H, Kondoh G, Fujii N, Kohno K, Nagasawa T. The essential functions of adipo-osteogenic progenitors as the hematopoietic stem and progenitor cell niche. Immunity. 2010;33:387–399. doi: 10.1016/j.immuni.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 20••.Zhou BO, Yue R, Murphy MM, Peyer JG, Morrison SJ. Leptin- receptor-expressing mesenchymal stromal cells represent the main source of bone formed by adult bone marrow. Cell Stem Cell. 2014;15:154–168. doi: 10.1016/j.stem.2014.06.008. They show that LepR+ perivascular stromal cells are the major source of osteoblasts and adipocytes in adult bones. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481:457–462. doi: 10.1038/nature10783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thompson Z, Miclau T, Hu D, Helms JA. A model for intramembranous ossification during fracture healing. J Orthop Res. 2002;20:1091–1098. doi: 10.1016/S0736-0266(02)00017-7. [DOI] [PubMed] [Google Scholar]
- 23.Hadjiargyrou M, O'Keefe RJ. The convergence of fracture repair and stem cells: interplay of genes, aging, environmental factors and disease. J Bone Miner Res. 2014;29:2307–2322. doi: 10.1002/jbmr.2373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24•.Colnot C. Skeletal cell fate decisions within periosteum and bone marrow during bone regeneration. J Bone Miner Res. 2009;24:274–282. doi: 10.1359/jbmr.081003. Using a lineage-tracing strategy on the basis of a transplantation-injury model, this study demonstrates for the first time that the periosteum and the bone marrow harbor stem cells with distinct properties. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25••.Rot C, Stern T, Blecher R, Friesem B, Zelzer E. A mechanical Jack-like Mechanism drives spontaneous fracture healing in neonatal mice. Dev Cell. 2014;31:159–170. doi: 10.1016/j.devcel.2014.08.026. This study shows that the neonatal periosteum can generate a bidirectional growth plate to realign fragments after angulated fracture, suggesting that periosteal stem cells have such capabilities. [DOI] [PubMed] [Google Scholar]
- 26.Ouyang Z, Chen Z, Ishikawa M, Yue X, Kawanami A, Leahy P, Greenfield EM, Murakami S. Prx1 and 3.2kb Col1a1 promoters target distinct bone cell populations in transgenic mice. Bone. 2013;58:136–145. doi: 10.1016/j.bone.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27•.Kawanami A, Matsushita T, Chan YY, Murakami S. Mice expressing GFP and CreER in osteochondro progenitor cells in the periosteum. Biochem Biophys Res Commun. 2009;386:477–482. doi: 10.1016/j.bbrc.2009.06.059. They show that Prrx1+ cells in the adult periosteum behave as osteo-chondroprogenitors that respond to the bone fracture. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28••.Matthews BG, Grcevic D, Wang L, Hagiwara Y, Roguljic H, Joshi P, Shin DG, Adams DJ, Kalajzic I. Analysis of alphaSMA-labeled progenitor cell commitment identifies notch signaling as an important pathway in fracture healing. J Bone Miner Res. 2014;29:1283–1294. doi: 10.1002/jbmr.2140. They show that aSMA+ periosteal cells proliferate in response to injury and contribute to chondrocytes and osteoblasts during fracture repair. [DOI] [PMC free article] [PubMed] [Google Scholar]