Abstract
Unlike mammals, zebrafish are able to regenerate a damaged retina. Key to this regenerative response are Müller glia that respond to retinal injury by undergoing a reprogramming event that allows them to divide and generate a retinal progenitor that is multipotent and responsible for regenerating all major retinal neuron types. The fish and mammalian retina are composed of similar cell types with conserved function. Because of this it is anticipated that studies of retina regeneration in fish may suggest strategies for stimulating Müller glia reprogramming and retina regeneration in mammals. In this review we describe recent advances and future directions in retina regeneration research using zebrafish as a model system.
Introduction
Tissue regeneration provides a way for restoring function to damaged and diseased tissues and organs, and for reversing the effects of aging. Mammals exhibit very little propensity for regeneration, although a limited amount does occur in tissues and organs like skin, skeletal muscle and liver. Other systems, like the central nervous system (CNS) do not regenerate in mammals and injuries or disease to the CNS generally result in irreparable damage.
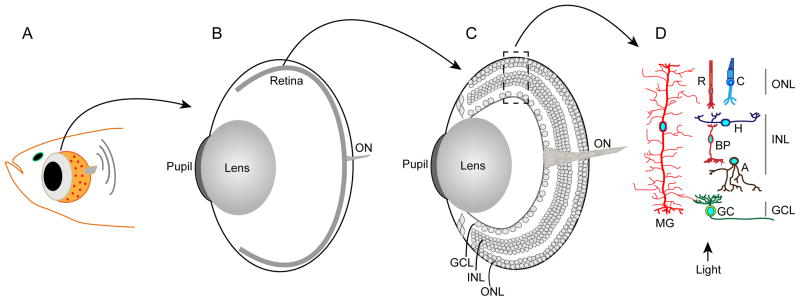
The retina lies at the back of the eye and is a relatively simple and accessible part of the CNS that is composed of 6 neural cell types and one glial cell type that are laminated into 3 distinct nuclear layers (Fig. 1). Because of this relatively simple composition and its accessibility to experimental manipulation the retina has served as an important experimental system for studying CNS regeneration. Furthermore, damaged neurons are the cause of blindness associated with retinal diseases like, macular degeneration, retinitis pigmentosa, diabetic retinopathies and glaucoma. Thus restoring these neurons in people suffering from blinding eye disease is a major goal of vision researchers. Unfortunately mammals are incapable of regenerating damaged retinal neurons. However, the robust capacity of zebrafish to regenerate all retinal cell types, combined with abundant genetic tools, puts this model system in a unique position to decipher the molecular mechanisms underlying retina regeneration. Furthermore, it is anticipated that an understanding of the mechanisms contributing to retina regeneration in fish will facilitate the development of strategies to stimulate this process in mammals. A number of excellent reviews have recently been written on retina regeneration in zebrafish and the reader is referred to these for a more comprehensive picture of retina regeneration1–3. In this review we will discuss the more recent literature relevant to retina regeneration in zebrafish, identify areas where information is missing and discuss how studies of retina regeneration in fish may be used to suggest strategies for stimulating retina regeneration in mammals.
Figure 1.
Retinal anatomy. Panel A: is a diagram of the fish head and eye. Panel B: is a diagram of the eye with the retina depicted as a thin piece of tissue lining the back of the eye. The optic nerve (ON) is composed of axons emanating from retinal ganglion cells. Panel C: is similar to panel B, but the retina is enlarged to show the various retinal layers. The retina is organized into three nuclear layers (outer nuclear layer [ONL], inner nuclear layer [INL] and ganglion cell layer [GCL]) that are separated by synaptic layers. Panel D: shows the neurons and glia of the retina. The ONL harbors Rod (R) and cone (C) photoreceptors. The INL harbors horizontal (H), bipolar (BP), amacrine (A) and Müller glial (MG) cells. The MG cell is unique in that it is the only retinal cell to extend processes into the ONL and GCL. The GCL harbors ganglion cells (GC) whose axons make up the optic nerve (ON).
Müller glia and their behavior in the damaged retina
Müller glia are the major glial cell type in the retina. They exhibit a radial morphology where their cell body and nuclei reside in the retina’s inner nuclear layer (INL) and they extend processes to the outer and inner retinal limiting membranes (Fig. 1). Müller glia also extend processes laterally allowing them to interact with neighboring neurons. This Müller glial cell architecture allows them to contribute to retinal structure and monitor the retinal environment so they can participate in retinal homeostasis and neural protection4,5.
Müller glia respond to retinal injury by hypertrophy and activation of cytoskeletal genes, like glial fibrillary acidic protein (Gfap) and vimentin6. Only rarely do Müller glia divide following retinal injury in mammals, and when they do they often result in fibrosis and glial scarring4. Interestingly, Müller glia in zebrafish respond to retinal injury by initiating a gliotic response that is characterized by hypertrophy and increased Gfap expression7. However, this gliotic response is transient in fish and accompanied by a reprogramming event that allows injury-responsive Müller glia to adopt properties of a retinal stem cell (Fig. 2)8,9. How Müller glia transition from a gliotic response to a regenerative one is not well understood and may be a key difference between regenerative success in fish and mammals. Thus, a better understanding of the mechanisms that drive gliosis in mammals and those that resolve it in fish may be useful in devising regenerative strategies in mammals.
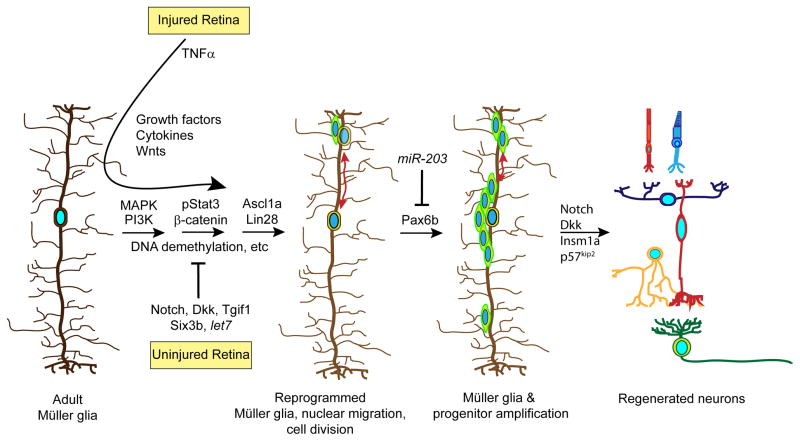
Figure 2.
Signaling pathways that regulate Müller glial cell reprogramming and retina regeneration. Shown are some of the pathways that have been identified to regulate Müller glia reprogramming, proliferation and progenitor differentiation. See text for details.
The acquisition of stem cell properties by fish Müller glia is associated with large scale changes in gene expression that precede Müller glia proliferation and the generation of retinal progenitors10–12. These changes in gene expression are accompanied by a partial reprogramming of the zebrafish genome that is characterized by changes in DNA methylation and activation of genes associated with multipotency (Fig. 2)8,9. The reprogrammed nuclei of injury-responsive Müller glia migrate from the INL to the outer nuclear layer (ONL) where they divide asymmetrically and then return to the INL (Fig. 2), a process referred to as interkinetic nuclear migration13,14. This asymmetric division results in the generation of a retinal progenitor that also exhibits interkinetic nuclear migration as it amplifies in a Pax6-dependent manner14,15, which results in a small population of progenitors capable of regenerating all major retinal neuron types (Fig. 2)16,17. The significance of interkinetic nuclear migration during retina regeneration has not been studied, but may expose nuclei to a changing gradient of factors spanning the retina that impact progenitor proliferation and differentiation18,19.
Signaling mechanisms underlying Müller glia reprogramming and retina regeneration
Retina regeneration is initiated by dying cells. These cells may inform Müller glial of their demise via secreted factors and/or through perturbations in their interaction with Müller glial cell processes. Interestingly, a variety of genes encoding growth factors and cytokines, like Hb-egf, Insulin, Igf-1, IL11, Leptin and Tnfα are induced in the injured fish retina and can stimulate Müller glia proliferation under appropriate conditions (Fig. 2)20–24. All of these factors are expressed by Müller glia-derived progenitors and therefore, may act in an autocrine and paracrine fashion to stimulate Müller glia and progenitor proliferation. It is worth mentioning that Müller glia have been shown to act as phagocytic cells by engulfing apoptotic photoreceptor cell bodies and this phagocytosis was necessary for Müller glia proliferation after injury7,25. The significance of this phagocytosis is not completely clear although it has been speculated that it may impact progenitor fate25.
Of the variety of secreted factors known to affect Müller glia proliferation, Tnfα is the only one shown to be expressed by dying cells and therefore, may represent a signal that communicates cell death to Müller glia23. However, Tnfα is not always associated with dying retinal neurons and on its own, has a very small effect on Müller glia proliferation23,24. We have shown that inhibition of Notch signaling significantly enhances Müller glia proliferation in the injured retina and that this is mediated by a MAPK-dependent signaling pathway20. Recent studies suggest that Notch inhibition in combination with Tnfα, stimulates Müller glia proliferation and neurogenic competency in the uninjured retina24.
Although inhibition of Notch signaling enhances Müller glia proliferation in the injured retina (Fig. 2)20,24, a number of studies have also indicated an increased expression of Notch signaling components in injury-responsive Müller glia20,26,27. One study suggests Notch signaling stimulates proliferation of progenitors destined to regenerate photoreceptors28, while another showed this signaling enhances progenitor differentiation20. We favor the idea that Notch signaling helps maintain Müller glia in a differentiated state and contributes to progenitor differentiation, while Notch signaling inhibition facilitates Müller glia reprogramming and proliferation in response to retinal cell death. This would imply constitutive Notch signaling in Müller glia of the uninjured retina and relief of this signaling in Müller glia responding to retinal injury. Surprisingly, Notch signaling has never been visualized in the fish retina and therefore, it is not clear that Notch signaling is restricted to Müller glia in the uninjured retina and specifically reduced in Müller glia-derived progenitors after retinal injury. Examining Notch signaling in Notch reporter fish will help resolve this issue29,30. Furthermore, signals regulating Notch signaling in the injured retina remain uncharacterized. The observation that Notch signaling contributes to Müller glia quiescence in the retina is similar to its role in maintaining neural stem cell quiescence in the adult brain31,32.
Some of the earliest signaling pathways regulated in the injured retina and necessary for Müller glial cell proliferation include MAPK and PI3K signaling (Fig. 2)20,22. Within the first hour following retinal injury, pErk and pAkt are increased in Müller glia and photoreceptor outer segments, respectively22. Interestingly, pErk returns to basal levels in Müller glia that proliferate. Although, the mechanisms by which these signaling pathways regulate retina regeneration deserves further attention, they are required for β-catenin and pStat3 expression that are necessary for injury-dependent Müller glia and progenitor proliferation21,22. The mechanism by which MAPK and PI3K signaling control β-catenin and pStat3 expression remains unclear.
MicroRNAs regulate mRNA stability and translation and have the potential to impact the expression of a large number of mRNAs during retina regeneration. Knockdown of the microRNA processing enzyme, DICER, has indicated microRNAs can stimulate Müller glia proliferation and retina regeneration33. Indeed, a number of microRNAs have been identified that increase in response to retinal injury, some of which contribute to progenitor amplification33. However, microRNA suppression also plays an important role in retina regeneration with let7 and miR-203 suppression contributing to Müller glia reprogramming and progenitor proliferation, respectively9,34. miR-203 appears to inhibit proliferation of Müller glia-derived progenitors by suppressing Pax6b expression (Fig. 2)34. A role for microRNAs in differentiation of progenitors in the injured retina remains unstudied, but is almost certain to have an important role.
Endogenous inhibitors of Müller glia reprogramming and retina regeneration
In addition, to positive acting factors that stimulate Müller glia reprogramming and proliferation in the injured retina, it is likely that the uninjured retinal environment will actively contribute to suppression of Müller glia reprogramming and proliferation (Fig. 2). Identifying these endogenous inhibitors of retina regeneration may not only inform us of mechanisms regulating retina regeneration in fish, but also provide candidates whose activity must be neutralized if we are to stimulate regeneration in mammals.
Previous studies have suggested that let7 microRNA expression is associated with cellular differentiation and that a Lin28-let7 signaling loop regulates decisions to adopt a stem cell or differentiated cell state35–37. Our studies suggest a similar signaling loop is operative in the zebrafish retina with Lin28 expression associated with Müller glia reprogramming and proliferation and let7 associated with Müller glia differentiation and quiescence9. Notch signaling also appears to be associated with quiescent Müller glia and progenitor differentiation in the injured retina20,24. This action appears to be mediated at least in part by Insm1a, which suppresses a genetic program driving progenitor proliferation10. Additional pathways contributing to Müller glia quiescence are indicated by fish harboring mutations in tgif1 and six3b, two genes that encode putative repressors of Tgfβ/Smad signaling. These genes are induced in the injured retina and mutations in these genes result in reduced proliferation of Müller glia in response to retinal injury38. This is reminiscent of BMP signaling in the adult mouse hippocampus where blockade of this signaling pathway recruits quiescent neural stem cells into the cell cycle39. Although Tgfβ/Smad signaling is proposed to contribute to Müller glia quiescence in zebrafish, this has never been directly tested; Tgif1 and Six3b have many targets in addition to the Tgfβ/Smad pathway and it is important to verify which targets mediate their effect on retina regeneration. Whether additional pathways contribute to Müller glia quiescence in the uninjured retina remains unknown; however, these putative pathways may be revealed by identifying genes that encode secreted factors whose expression is suppressed following retinal injury.
Extrinsic signals regulating lineage specification of Müller glia derived progenitors
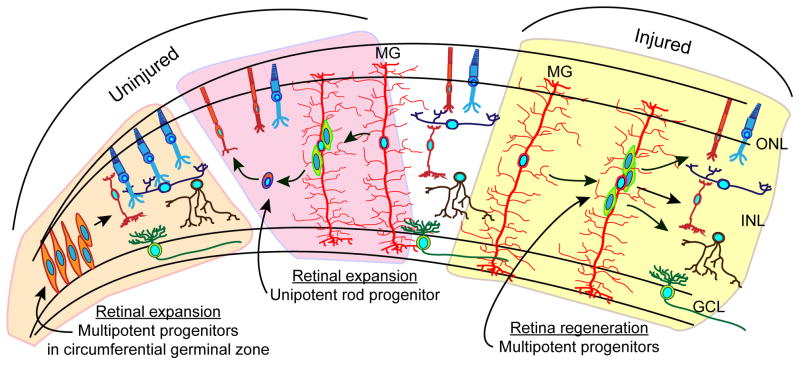
As the fish grows throughout life so does the retina. This retinal expansion results from the addition of new neurons seeded into the retinas circumferential germinal zone from neuroepithelial progenitors residing at the ciliary margin (Fig. 3)40. These progenitors give rise to all major retinal cell types except rods, which are generated by rod progenitors found in the ONL. Interestingly, besides being a source of multipotent progenitors in the injured retina, Müller glia are also the source of unipotent rod progenitors in the uninjured retina (Fig. 3)17,41,42. These unipotent progenitors are responsible for generating rods in the ONL as the retina expands throughout the fish’s life. Although unipotent and multipotent Müller glia-derived progenitors appear to be exclusively produced in uninjured and injured retinal environments, respectively, the particular aspects of these environments that drive Müller glia-derived progenitors to adopt unipotent or multipotent potent character remain poorly understood. Interestingly, growth factors and cytokines, like Hb-egf, insulin, Tnfα and leptin are induced in injury-responsive Müller glia and stimulate Müller glia in the uninjured retina to divide and produce multipotent progenitors20–24. Thus, these factors may be components of the injured retinal environment that contribute to progenitor multipotency. Other components of the uninjured and injured retinal environment that control the generation of multipotent progenitors remain to be defined. Identifying these environmental components, the signal transduction machinery and gene expression programs that drive progenitors to adopt unipotent or multipotent characteristics will be important for not only understanding Müller glia plasticity, but also for harnessing this plasticity for retinal repair.
Figure 3.
Retinal progenitors. Progenitors in the retinal periphery are responsible for producing all neurons except rods in the constantly expanding fish retina. Muller glia-derived unipotent rod progenitors are responsible for generating rods in the ONL of the expanding retina. Muller glial-derived multipotent progenitors are generated in the injured retina and responsible for regenerating all of the major retinal neurons.
Although injury-induced Müller-glia derived progenitors have the potential to regenerate all major retinal cell types, some reports suggest they predominantly regenerate ablated neurons41,43,44. Other studies suggest progenitors in the injured retina may retain a multipotent character regardless of the type of neuron ablated since ectopic neurons appear outside the injury site and are also found in uninjured retinas treated with a variety of growth factors and cytokines20–22,24. Recent studies suggest Müller glia are intrinsically multipotent regardless of the type of neuron ablated and that ectopic or excess neurons are generated45. Thus, it is important to determine if these additional neurons persist in the retina and contribute to retina structure and function.
Conclusions and future directions
The zebrafish retina provides an ideal system for studying retina regeneration. Investigations into the mechanisms underlying retina regeneration have revealed gene expression programs and signaling pathways that are important for this process. Comparison of retinal injury responses in zebrafish and mammals may help identify factors that can stimulate mammalian retina regeneration. The study of retina regeneration is still in its infancy and there remain many unanswered questions. Some were described above, additional questions are outlined below.
Are all Müller glia equal in their ability to generate progenitors?
How do dying cells communicate to Müller glia to stimulate their reprogramming and proliferation?
How much of regeneration is a recapitulation of development and what are its unique aspects?
What directs Müller glia-derived progenitors to regenerate specific cell types? Is there heterogeneity between progenitors associated with a particular Müller glia?
Why is progenitor proliferation limited? How is it controlled? What are the consequences of reducing or enhancing this proliferation?
What components of an uninjured and injured retinal environment direct Müller glia to generate unipotent and multipotent progenitors, respectively?
What is the hierarchical and regulatory relationship of various signaling cascades, like MAPK, PI3K, Notch, β-catenin and Stat3 that regulate retina regeneration?
Acknowledgments
DG and JW are supported by NEI grant RO1 EY 018132 from the NIH, DG is also the recipient of a Research to Prevent Blindness Innovative Ophthalmic Research Award and gifts from the Marjorie and Maxwell Jospey Foundation and the Shirlye and Peter Helman Fund. We are grateful to the members of the Goldman laboratory for their discussions and feedback on this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
Papers of particular interest, published within the period of review have been highlighted as
• of special interest
•• of outstanding interest
- 1.Goldman D. Muller glial cell reprogramming and retina regeneration. Nature reviews Neuroscience. 2014;15:431–42. doi: 10.1038/nrn3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenkowski JR, Raymond PA. Muller glia: Stem cells for generation and regeneration of retinal neurons in teleost fish. Prog Ret Eye Res. 2014;40:94–123. doi: 10.1016/j.preteyeres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorsuch RA, Hyde DR. Regulation of Muller glial dependent neuronal regeneration in the damaged adult zebrafish retina. Exp Eye Res. 2014;123:131–40. doi: 10.1016/j.exer.2013.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bringmann A, et al. Cellular signaling and factors involved in Muller cell gliosis: neuroprotective and detrimental effects. Prog Retin Eye Res. 2009;28:423–51. doi: 10.1016/j.preteyeres.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Reichenbach A, Bringmann A. New functions of Muller cells. Glia. 2013;61:651–78. doi: 10.1002/glia.22477. [DOI] [PubMed] [Google Scholar]
- 6.Hippert C, et al. Muller glia activation in response to inherited retinal degeneration is highly varied and disease-specific. PLoS One. 2015;10:e0120415. doi: 10.1371/journal.pone.0120415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •7.Thomas JL, Ranski AH, Morgan GW, Thummel R. Reactive gliosis in the adult zebrafish retina. Exp Eye Res. 2016;143:98–109. doi: 10.1016/j.exer.2015.09.017. In this paper the authors provide evidence that Muller glia exhibit a transient gliotic response following retinal injury. [DOI] [PubMed] [Google Scholar]
- •8.Powell C, Grant AR, Cornblath E, Goldman D. Analysis of DNA methylation reveals a partial reprogramming of the Muller glia genome during retina regeneration. Proc Natl Acad Sci U S A. 2013;110:19814–9. doi: 10.1073/pnas.1312009110. This is the first demonstration of reprogramming of the Muller glia genome by changes in DNA methylation following retinal injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••9.Ramachandran R, Fausett BV, Goldman D. Ascl1a regulates Muller glia dedifferentiation and retinal regeneration through a Lin-28-dependent, let-7 microRNA signalling pathway. Nat Cell Biol. 2010a;12:1101–7. doi: 10.1038/ncb2115. This paper shows that Muller glia reprogramming and adoption of stem cell properties shares signaling pathways with mammalian fibroblasts that are reprogrammed to pluripotency. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •10.Ramachandran R, Zhao XF, Goldman D. Insm1a-mediated gene repression is essential for the formation and differentiation of Muller glia-derived progenitors in the injured retina. Nat Cell Biol. 2012;14:1013–23. doi: 10.1038/ncb2586. This paper identifies a transcriptional repressor that controls progenitor cell cycle exit and limits the number of progenitors in the injured retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Qin Z, Barthel LK, Raymond PA. Genetic evidence for shared mechanisms of epimorphic regeneration in zebrafish. Proc Natl Acad Sci U S A. 2009;106:9310–5. doi: 10.1073/pnas.0811186106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kassen SC, et al. Time course analysis of gene expression during light-induced photoreceptor cell death and regeneration in albino zebrafish. Dev Neurobiol. 2007;67:1009–31. doi: 10.1002/dneu.20362. [DOI] [PubMed] [Google Scholar]
- •13.Nagashima M, Barthel LK, Raymond PA. A self-renewing division of zebrafish Muller glial cells generates neuronal progenitors that require N-cadherin to regenerate retinal neurons. Development. 2013;140:4510–21. doi: 10.1242/dev.090738. This study indicated assymetric division of Muller glia in response to retinal injury. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lahne M, Li J, Marton RM, Hyde DR. Actin-Cytoskeleton- and Rock-Mediated INM Are Required for Photoreceptor Regeneration in the Adult Zebrafish Retina. J Neurosci. 2015;35:15612–34. doi: 10.1523/JNEUROSCI.5005-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •15.Thummel R, et al. Pax6a and Pax6b are required at different points in neuronal progenitor cell proliferation during zebrafish photoreceptor regeneration. Exp Eye Res. 2010;90:572–82. doi: 10.1016/j.exer.2010.02.001. This paper reports that Pax6 transcription factors control progenitor amplification during retina regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramachandran R, Reifler A, Parent JM, Goldman D. Conditional gene expression and lineage tracing of tuba1a expressing cells during zebrafish development and retina regeneration. J Comp Neurol. 2010;518:4196–4212. doi: 10.1002/cne.22448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fausett BV, Goldman D. A role for alpha1 tubulin-expressing Muller glia in regeneration of the injured zebrafish retina. J Neurosci. 2006;26:6303–13. doi: 10.1523/JNEUROSCI.0332-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taverna E, Huttner WB. Neural progenitor nuclei IN motion. Neuron. 2010;67:906–14. doi: 10.1016/j.neuron.2010.08.027. [DOI] [PubMed] [Google Scholar]
- 19.Lahne M, Hyde DR. Interkinetic Nuclear Migration in the Regenerating Retina. Adv Exp Med Biol. 2016;854:587–93. doi: 10.1007/978-3-319-17121-0_78. [DOI] [PubMed] [Google Scholar]
- ••20.Wan J, Ramachandran R, Goldman D. HB-EGF is necessary and sufficient for Müller glia dedifferentiation and retina regeneration. Dev Cell. 2012;22:334–347. doi: 10.1016/j.devcel.2011.11.020. This was the first report of Notch and MAPK signaling regulating Muller glia proliferation in the injured fish retina. It was also the first to report a growth factor that was capable of stimulating Muller glia proliferation in the uninjured retina. Furthermore, HB-EGF was expressed by injury responsive Muller glia suggesting an autocrine mechanism contributes to Muller glia proliferation in the injured retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •21.Zhao XF, et al. Leptin and IL-6 Family Cytokines Synergize to Stimulate Muller Glia Reprogramming and Retina Regeneration. Cell Reports. 2014;9:272–284. doi: 10.1016/j.celrep.2014.08.047. This study reports on the identification of cytokines expressed in injury responsive Muller glia and acting via gp130 coupled Jak/Stat signaling pathway to regulate Muller glia proliferation and retina regeneration. This study also suggests an autocrine mechanism contributing to retina regeneration. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••22.Wan J, Zhao XF, Vojtek A, Goldman D. Retinal Injury, Growth Factors, and Cytokines Converge on beta-Catenin and pStat3 Signaling to Stimulate Retina Regeneration. Cell Reports. 2014;9:285–297. doi: 10.1016/j.celrep.2014.08.048. In this paper Wan et al., provide evidence that MAPK and PI3K signaling act upstream of Jak/Stat and beta-catenin signaling to stimulate Muller glia proliferation in the injured retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••23.Nelson CM, et al. Tumor necrosis factor-alpha is produced by dying retinal neurons and is required for Muller glia proliferation during zebrafish retinal regeneration. J Neurosci. 2013;33:6524–39. doi: 10.1523/JNEUROSCI.3838-12.2013. This study identifies a signal emanating from dying cells that may stimulate Muller glia proliferation in the injured retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ••24.Conner C, Ackerman KM, Lahne M, Hobgood JS, Hyde DR. Repressing notch signaling and expressing TNFalpha are sufficient to mimic retinal regeneration by inducing Muller glial proliferation to generate committed progenitor cells. J Neurocsci. 2014;34:14403–19. doi: 10.1523/JNEUROSCI.0498-14.2014. This report shows that TNFa is more effective at stimulating Muller glia proliferation in the uninjured retina if Notch signaling is also inhibited. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bailey TJ, Fossum SL, Fimbel SM, Montgomery JE, Hyde DR. The inhibitor of phagocytosis, O-phospho-l-serine, suppresses Muller glia proliferation and cone cell regeneration in the light-damaged zebrafish retina. Exp Eye Res. 2010;91:601–612. doi: 10.1016/j.exer.2010.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Raymond PA, Barthel LK, Bernardos RL, Perkowski JJ. Molecular characterization of retinal stem cells and their niches in adult zebrafish. BMC Dev Biol. 2006;6:36. doi: 10.1186/1471-213X-6-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yurco P, Cameron DA. Cellular correlates of proneural and Notch-delta gene expression in the regenerating zebrafish retina. Vis Neurosci. 2007;24:437–43. doi: 10.1017/S0952523807070496. [DOI] [PubMed] [Google Scholar]
- 28.Taylor SM, et al. The bHLH Transcription Factor NeuroD Governs Photoreceptor Genesis and Regeneration Through Delta-Notch Signaling. Invest Ophthalmol Vis Sci. 2015;56:7496–515. doi: 10.1167/iovs.15-17616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parsons MJ, et al. Notch-responsive cells initiate the secondary transition in larval zebrafish pancreas. Mech Dev. 2009;126:898–912. doi: 10.1016/j.mod.2009.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ninov N, Borius M, Stainier DY. Different levels of Notch signaling regulate quiescence, renewal and differentiation in pancreatic endocrine progenitors. Development. 2012;139:1557–67. doi: 10.1242/dev.076000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alunni A, et al. Notch3 signaling gates cell cycle entry and limits neural stem cell amplification in the adult pallium. Development. 2013;140:3335–47. doi: 10.1242/dev.095018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chapouton P, et al. Notch activity levels control the balance between quiescence and recruitment of adult neural stem cells. J Neurosci. 2010;30:7961–74. doi: 10.1523/JNEUROSCI.6170-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rajaram K, Harding RL, Bailey T, Patton JG, Hyde DR. Dynamic miRNA expression patterns during retinal regeneration in zebrafish: reduced dicer or miRNA expression suppresses proliferation of Muller glia-derived neuronal progenitor cells. Dev Dyn. 2014;243:1591–605. doi: 10.1002/dvdy.24188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- •34.Rajaram K, Harding RL, Hyde DR, Patton JG. miR-203 regulates progenitor cell proliferation during adult zebrafish retina regeneration. Dev Biol. 2014;392:393–403. doi: 10.1016/j.ydbio.2014.05.005. This report suggests miR-203 acts on Pax6 expression to regulate progenitor proliferation in the injured retina. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melton C, Judson RL, Blelloch R. Opposing microRNA families regulate self-renewal in mouse embryonic stem cells. Nature. 2010;463:621–626. doi: 10.1038/nature08725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nimmo RA, Slack FJ. An elegant miRror: microRNAs in stem cells, developmental timing and cancer. Chromosoma. 2009;118:405–18. doi: 10.1007/s00412-009-0210-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rybak A, et al. A feedback loop comprising lin-28 and let-7 controls pre-let-7 maturation during neural stem-cell commitment. Nat Cell Biol. 2008;10:987–93. doi: 10.1038/ncb1759. [DOI] [PubMed] [Google Scholar]
- 38.Lenkowski JR, et al. Retinal regeneration in adult zebrafish requires regulation of TGFbeta signaling. Glia. 2013;61:1687–97. doi: 10.1002/glia.22549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mira H, et al. Signaling through BMPR-IA regulates quiescence and long-term activity of neural stem cells in the adult hippocampus. Cell Stem Cell. 2010;7:78–89. doi: 10.1016/j.stem.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 40.Otteson DC, Hitchcock PF. Stem cells in the teleost retina: persistent neurogenesis and injury-induced regeneration. Vision Res. 2003;43:927–36. doi: 10.1016/s0042-6989(02)00400-5. [DOI] [PubMed] [Google Scholar]
- 41.Fimbel SM, Montgomery JE, Burket CT, Hyde DR. Regeneration of inner retinal neurons after intravitreal injection of ouabain in zebrafish. J Neurosci. 2007;27:1712–24. doi: 10.1523/JNEUROSCI.5317-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bernardos RL, Barthel LK, Meyers JR, Raymond PA. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J Neurosci. 2007;27:7028–40. doi: 10.1523/JNEUROSCI.1624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fraser B, DuVal MG, Wang H, Allison WT. Regeneration of cone photoreceptors when cell ablation is primarily restricted to a particular cone subtype. PLoS One. 2013;8:e55410. doi: 10.1371/journal.pone.0055410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hochmann S, et al. Fgf Signaling is Required for Photoreceptor Maintenance in the Adult Zebrafish Retina. PLoS One. 2012;7:e30365. doi: 10.1371/journal.pone.0030365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Powell C, Cornblath E, Elsaeidi F, Wan J, Goldman D. Zebrafish Müller glia-derived progenitors are multipotent, exhibit proliferative biases and regenerate excess neurons. Sci Rep. 2016;6:24851. doi: 10.1038/srep24851. [DOI] [PMC free article] [PubMed] [Google Scholar]