Figure 3. Schema of the First-In-Human Phase 1 Study of Adoptive Immunotherapy with Allogeneic Memory-Like NK Cells.
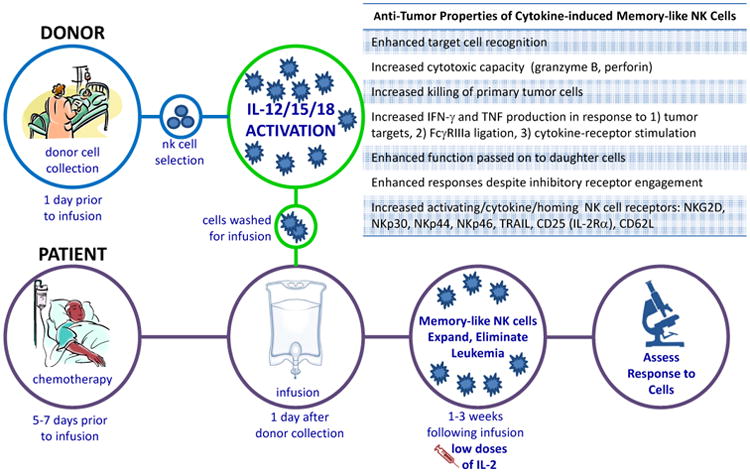
Patients with relapsed or refractory acute myeloid leukemia (AML) are treated with chemotherapy to provide immunosuppression that allows the engraftment of allogeneic donor NK cells. Related donors undergo leukapheresis to collect peripheral blood mononuclear cells, and NK cells are purified by CD3 negative and CD56 positive selection (CliniMACs device that results in >90% CD56+CD3- NK cells). These purified NK cells are then pre-activated with IL-12, IL-15, and IL-18 for 12-16 hours in a GMP setting. Cells are extensively washed to remove cytokines and infused into the cancer patient. Memory-like NK cell differentiate and expand, peaking 1-2 weeks post infusion, supported by low dose IL-2 therapy. Clinical responses are assessed at day 14 and 30 post-infusion using international working group criteria. The anti-tumor properties of cytokine-induced memory-like NK cells are summarized in the box inset.
