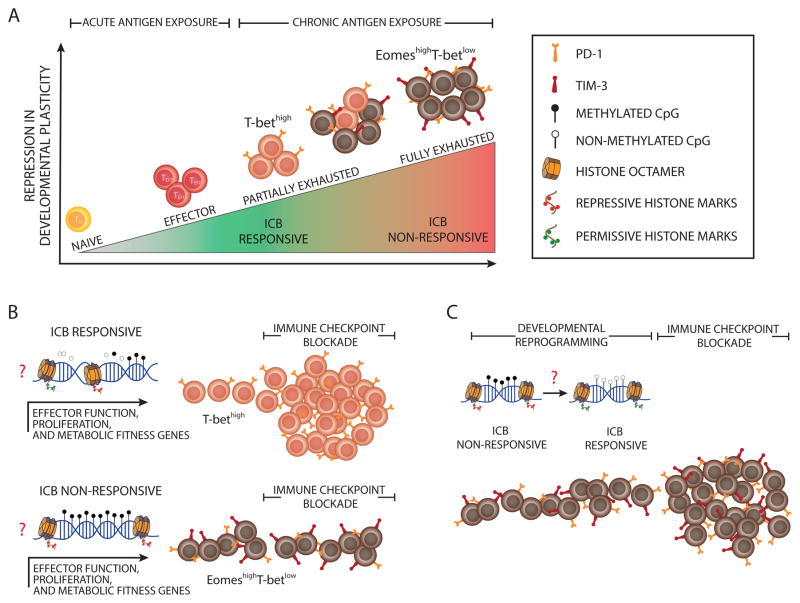
Key Figure, Figure 1. Epigenetic Barriers to Immune Checkpoint Blockade-Mediated Rejuvenation of T Cells.
(A) The schematic depicts the repression in developmental plasticity acquired by antigen-specific T cells during clonal expansion in response to acute or chronic antigen exposure. Upon acute antigen exposure, naive CD8+ T cells clonally expand and differentiate into effector cells. During chronic high-level antigen exposure, persistent T cell receptor (TCR) stimulation can cause T cell exhaustion. Effector function is progressively repressed during the development of T cell exhaustion, thereby leading to a heterogeneous population of T cells at various levels of exhaustion. Partially exhausted T cells are phenotypically defined by sustained expression of a minimal number of immune inhibitory receptors (IRs) and characterized by differential expression of T-bet and Eomes (T-bethigh Eomeslow T cells), whereas fully exhausted T cells are marked by co-expression of multiple IRs. The immune checkpoint blockade (ICB) obstructs inhibitory signals from immune IRs, resulting in rejuvenation of partially exhausted T cells. (B) The schematic depicts the effect of progressive epigenetic programming that reinforces silencing of genes involved in effector function, proliferation, and metabolic fitness of exhausted T cells. Partially exhausted T cells may retain a degree of epigenetic plasticity at exhaustion-specific silenced genes manifested by partially methylated DNA and deposition of fewer repressive, more permissive histone marks. Terminally differentiated exhausted T cells may be more epigenetically restrained through fully methylated DNA, the deposition of more repressive histone marks, and the loss of permissive histone marks. (C) The diagram models the potential reprogramming of exhaustion-associated epigenetic programs that could be done to remove cell-intrinsic barriers to achieving a better, possibly durable rejuvenation response of fully exhausted T cells following ICB therapy.