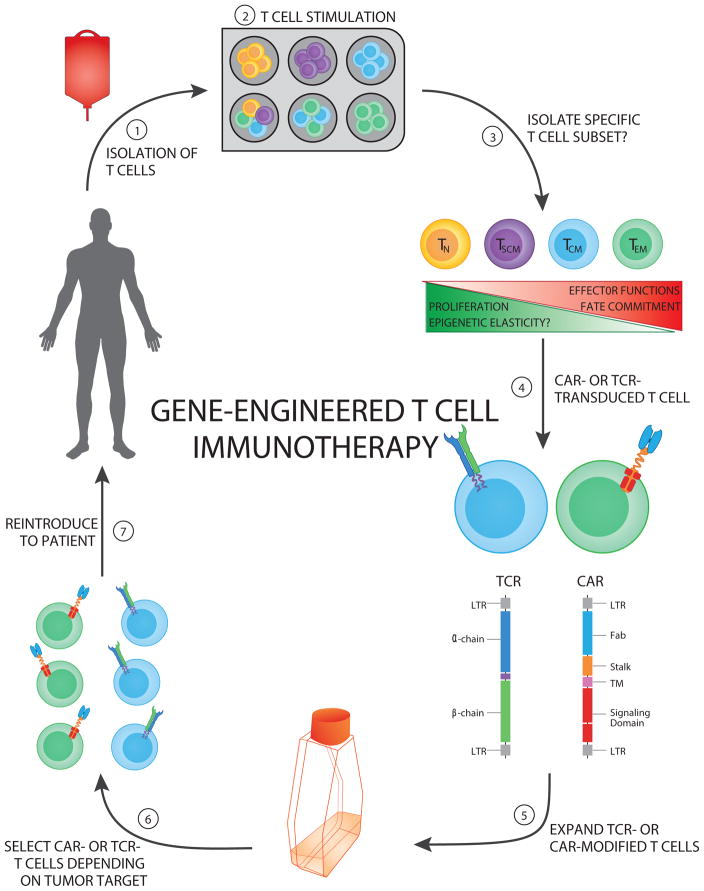
Figure 2. Genetically-Engineered T cell Immunotherapeutic Approaches.
The schematic depicts the multiuse process of generating genetically engineered human T cells targeted against specific tumor antigens.
(Step 1) Autologous T cells can be isolated from a patient’s peripheral blood mononuclear cells or an excised tumor.
(Step 2) T cell pools or specific subsets can be stimulated with (1) activating CD3 antibody (OKT3), (2) anti-CD3/CD28–coated beads, or (3) allogeneic feeder cell lines.
(Step 3) Specific T cell subsets can be sorted or enriched according to their differentiation status to exploit proliferative and effector functions.
(Step 4) Engineered T cells can be generated by cloning either T cell receptors (TCRs) or chimeric antigen receptors (CARs) and transducing a patient’s T cells with retro- or lenti-viruses, thus redirecting recognition toward tumor-associated antigens.
(Step 5) Engineered T cells can then be expanded in vitro in the presence of conditioning cytokines (e.g., IL-2 or IL-15) to increase the frequency of tumor-specific T cells generated.
(Step 6) Engineered T cells specific for an antigen (target) of interest are selected.
(Step 7) Engineered T cells can then be reinfused into the patient (usually post-lymphodepletion).