Abstract
We have identified two distinct transcripts of inositol 1,4,5-trisphosphate receptor by using the PCR on first-strand cDNAs from various rat tissues. The longer form, corresponding to the previously cloned adult rat brain inositol 1,4,5-trisphosphate receptor, contains a 120-nucleotide insert between the two cAMP-dependent protein kinase phosphorylation consensus sequences. The shorter form (lacking the insert) predominates in fetal brain and peripheral tissues and appears to represent a nonneuronal receptor, whereas the longer form is found in adult brain and appears to be exclusively neuronal. The phosphorylation kinetics by cAMP-dependent protein kinase and the phosphopeptide maps differ for inositol 1,4,5-trisphosphate receptors purified from tissues predominantly expressing different forms of the transcript.
Full text
PDF

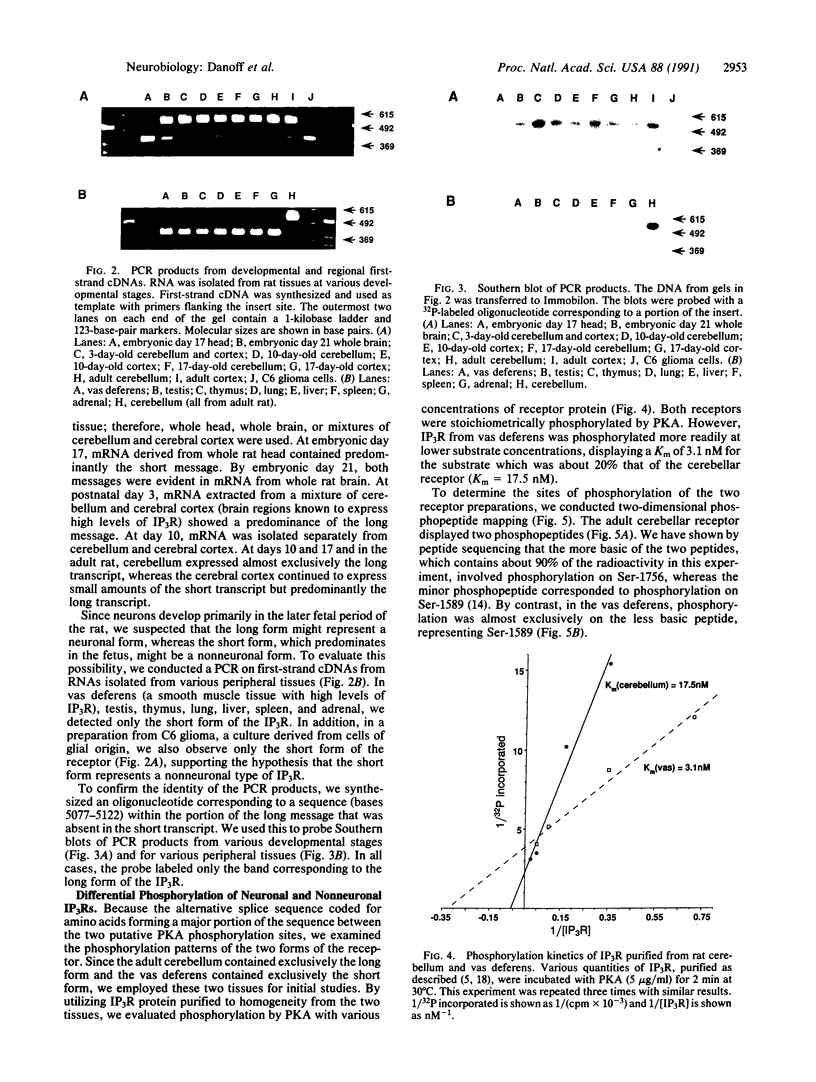

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berridge M. J., Irvine R. F. Inositol phosphates and cell signalling. Nature. 1989 Sep 21;341(6239):197–205. doi: 10.1038/341197a0. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Brugge J., Cotton P., Lustig A., Yonemoto W., Lipsich L., Coussens P., Barrett J. N., Nonner D., Keane R. W. Characterization of the altered form of the c-src gene product in neuronal cells. Genes Dev. 1987 May;1(3):287–296. doi: 10.1101/gad.1.3.287. [DOI] [PubMed] [Google Scholar]
- Danoff S. K., Supattapone S., Snyder S. H. Characterization of a membrane protein from brain mediating the inhibition of inositol 1,4,5-trisphosphate receptor binding by calcium. Biochem J. 1988 Sep 15;254(3):701–705. doi: 10.1042/bj2540701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Bredt D. S., Cameron A. M., Snyder S. H. Inositol trisphosphate receptor: phosphorylation by protein kinase C and calcium calmodulin-dependent protein kinases in reconstituted lipid vesicles. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2232–2235. doi: 10.1073/pnas.88.6.2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Snyder S. H. Calcium flux mediated by purified inositol 1,4,5-trisphosphate receptor in reconstituted lipid vesicles is allosterically regulated by adenine nucleotides. Proc Natl Acad Sci U S A. 1990 Mar;87(6):2147–2151. doi: 10.1073/pnas.87.6.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris C. D., Huganir R. L., Supattapone S., Snyder S. H. Purified inositol 1,4,5-trisphosphate receptor mediates calcium flux in reconstituted lipid vesicles. Nature. 1989 Nov 2;342(6245):87–89. doi: 10.1038/342087a0. [DOI] [PubMed] [Google Scholar]
- Furuichi T., Yoshikawa S., Miyawaki A., Wada K., Maeda N., Mikoshiba K. Primary structure and functional expression of the inositol 1,4,5-trisphosphate-binding protein P400. Nature. 1989 Nov 2;342(6245):32–38. doi: 10.1038/342032a0. [DOI] [PubMed] [Google Scholar]
- Ghosh T. K., Mullaney J. M., Tarazi F. I., Gill D. L. GTP-activated communication between distinct inositol 1,4,5-trisphosphate-sensitive and -insensitive calcium pools. Nature. 1989 Jul 20;340(6230):236–239. doi: 10.1038/340236a0. [DOI] [PubMed] [Google Scholar]
- Huganir R. L., Miles K., Greengard P. Phosphorylation of the nicotinic acetylcholine receptor by an endogenous tyrosine-specific protein kinase. Proc Natl Acad Sci U S A. 1984 Nov;81(22):6968–6972. doi: 10.1073/pnas.81.22.6968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez R., Mathey-Prevot B., Bernards A., Baltimore D. Neuronal pp60c-src contains a six-amino acid insertion relative to its non-neuronal counterpart. Science. 1987 Jul 24;237(4813):411–415. doi: 10.1126/science.2440106. [DOI] [PubMed] [Google Scholar]
- Mignery G. A., Newton C. L., Archer B. T., 3rd, Südhof T. C. Structure and expression of the rat inositol 1,4,5-trisphosphate receptor. J Biol Chem. 1990 Jul 25;265(21):12679–12685. [PubMed] [Google Scholar]
- Mignery G. A., Südhof T. C. The ligand binding site and transduction mechanism in the inositol-1,4,5-triphosphate receptor. EMBO J. 1990 Dec;9(12):3893–3898. doi: 10.1002/j.1460-2075.1990.tb07609.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mourey R. J., Verma A., Supattapone S., Snyder S. H. Purification and characterization of the inositol 1,4,5- trisphosphate receptor protein from rat vas deferens. Biochem J. 1990 Dec 1;272(2):383–389. doi: 10.1042/bj2720383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Studies and perspectives of protein kinase C. Science. 1986 Jul 18;233(4761):305–312. doi: 10.1126/science.3014651. [DOI] [PubMed] [Google Scholar]
- Pyper J. M., Bolen J. B. Identification of a novel neuronal C-SRC exon expressed in human brain. Mol Cell Biol. 1990 May;10(5):2035–2040. doi: 10.1128/mcb.10.5.2035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimann E. M., Beham R. A. Catalytic subunit of cAMP-dependent protein kinase. Methods Enzymol. 1983;99:51–55. doi: 10.1016/0076-6879(83)99039-0. [DOI] [PubMed] [Google Scholar]
- Ross C. A., Meldolesi J., Milner T. A., Satoh T., Supattapone S., Snyder S. H. Inositol 1,4,5-trisphosphate receptor localized to endoplasmic reticulum in cerebellar Purkinje neurons. Nature. 1989 Jun 8;339(6224):468–470. doi: 10.1038/339468a0. [DOI] [PubMed] [Google Scholar]
- Supattapone S., Danoff S. K., Theibert A., Joseph S. K., Steiner J., Snyder S. H. Cyclic AMP-dependent phosphorylation of a brain inositol trisphosphate receptor decreases its release of calcium. Proc Natl Acad Sci U S A. 1988 Nov;85(22):8747–8750. doi: 10.1073/pnas.85.22.8747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Supattapone S., Worley P. F., Baraban J. M., Snyder S. H. Solubilization, purification, and characterization of an inositol trisphosphate receptor. J Biol Chem. 1988 Jan 25;263(3):1530–1534. [PubMed] [Google Scholar]
- Worley P. F., Baraban J. M., Supattapone S., Wilson V. S., Snyder S. H. Characterization of inositol trisphosphate receptor binding in brain. Regulation by pH and calcium. J Biol Chem. 1987 Sep 5;262(25):12132–12136. [PubMed] [Google Scholar]