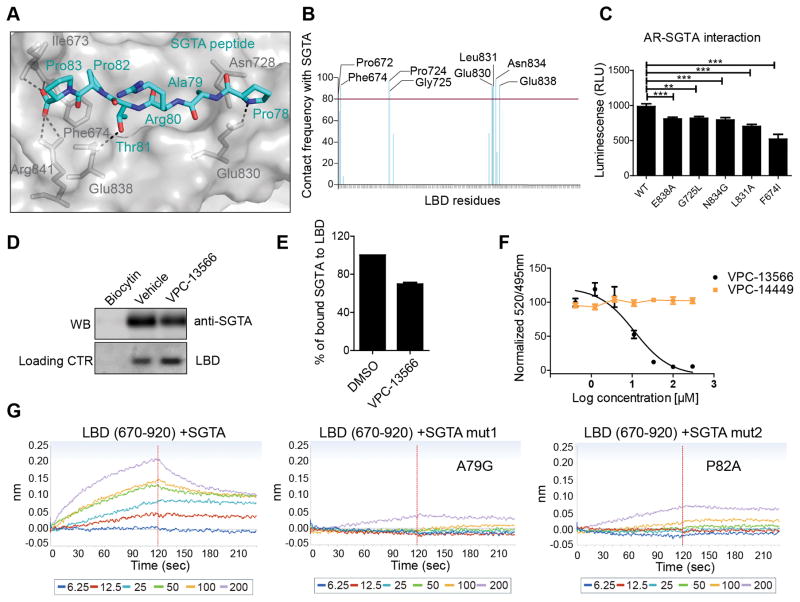
Figure 4. The binding of SGTA to the BF3 pocket of AR.
A- The most common MD simulations binding conformation of SGTA peptide (cyan) into the BF3 pocket (grey). Critical BF3 residues that make H-bond interactions (Dotted lines) are highlighted (grey sticks). B- BF3 residues that interact with the PARTPP peptide more than 80% of the MD simulations time. C-Mutations in the BF3 pocket affect SGTA-AR interaction in mammalian two hybrid assay. D–E Pull down of SGTA protein by a purified AR-LBD protein (residues 670-920) and the effect of 100 μM VPC-13566 on LBD/SGTA interaction. F- Displacement of a FITC-SGTA peptide from the BF3 pocket by increasing concentrations of VPC-13566 using a TR-FRET assay. G- The direct binding of SGTA peptide containing the PARTPP motif to a purified LBD using BLI. Mutating A79 or P82 of this motif disrupted the LBD-peptide interaction.