Abstract
Background:
Higher than expected cardiovascular mortality in hemodialysis patients, has been attributed to dyslipidemia as well as inflammation. Beta2-Microglobulin (β2M) is an independent predictor of outcome for hemodialysis patients and a representative substance of middle molecules.
Results:
In 40 patients in high-flux membrane hemodialysis, we found negative correlation of β2M with high density lipoprotein (r=-0.73, p<0.001) and albumin (r= -0.53, p<0.001) and positive correlation with triglycerides (r=0.69, p<0.001), parathyroid hormone (r=0.58, p < 0.05) and phosphorus (r= 0.53, p<0.001). There was no correlation of β2M with C- reactive protein (CRP) and interleukin-6 (IL-6). During the follow-up period of three years, 6 out of 40 patients have died from cardiovascular events.
Conclusion:
In high-flux membrane hemodialysis patients, we observed a significant relationship of β2M with dyslipidemia and mineral bone disorders, but there was no correlation with inflammation.
Keywords: hemodialysis, beta-2 microglobulin, inflammation, dyslipidemia, cardiovascular diseases
1. INTRODUCTION
Hemodialysis (HD) patients have high mortality rate (1). Uremia related, non -traditional risk factors, such as inflammation, oxidative stress, dyslipidemia, vascular calcification alterations in calcium and phosphorus (P) metabolism, have been proposed to play a central role (2). Elevated plasma beta2 microglobulin (β2M), is a well-known characteristic of chronic renal failure. Predialysis serum β2M level predicted mortality and increase of β2M clearance during HD was associated with improved outcomes (3). The source of the elevated serum β2M in hemodialysis patients, has not been explained absolutely. There is controversy as to whether elevated levels are caused predominantly by increased synthesis, the use of membranes in hemodialysis with different clearance capacities, or diminished renal elimination. Use of middle and high-flux biocompatible membranes was shown to be associated with a notable reduction β2M (4). β2M has been shown to be elevated in chronic inflammations. The surface of lymphocytes and monocytes are particularly rich in β2M, the latter being synthesized to large amounts by lymphocytes and regulated by interferons and proinflammatory cytokines (5), which might explain the pathophysiological “role” in atherosclerosis. But remains to be further clarified if β2M is solely a marker of inflammation or if it has a direct pathogenic effect and if other yet unknown confounders may influence β2M levels (6).
Systemic inflammation is a common complication in HD patients. The uremic state is associated with an altered immune response, and intermittent stimulation by endotoxins originating from the dialysis water supply and artificial vein grafts or bio incompatibility caused the increased circulating inflammatory proteins, such as C-reactive protein (CRP) (7), produced by the liver, mainly in response to interleukin -6 (IL-6).
Hemodialysis patients, exhibit significant alterations in lipoprotein metabolism, which in their most advanced form result in the development of severe dyslipidemia. Lipid disorders stem largely from dysregulation of high density lipoprotein (HDL) and triglyceride-rich lipoproteins metabolism. The down regulation of the expression of several genes along with the changes in the composition of lipoprotein particles and the direct inhibitory effect of various uremic ‘toxins’ on the enzymes involved in lipid metabolism, represent the most important pathophysiological mechanisms underlying the development of hypertriglyceridemia (8). Low density lipoprotein (LDL) levels are usually not elevated. Several mechanisms, working in concert, may underlie the reduction in HDL levels, which is usually indicative of impaired reverse cholesterol transport. Specifically, maturation of HDL is impaired and its composition is altered. Thus, uremic patients exhibit decreased levels of apolipoproteins AI and AII, diminished activity of lecithin-cholesterol acyl-transferase, as well as increased activity of cholesteryl ester transfer protein that facilitates the transfer of cholesterol esters from HDL to triglyceride-rich lipoproteins, thus reducing the concentration of HDL (8).
Lipoprotein(a) [Lp(a)] is an independent risk factor for clinical events attributed to atherosclerotic cardiovascular disease in chronic hemodialysis. Lp(a) levels are frequently elevated in HD patients (9) and are considered a major risk factor for cardiovascular disease (10).
Secondary hyperparathyroidism, is one of the major complications of patients in chronic hemodialysis (11). Parathyroid hormone (PTH) starts to rise very early in the course of kidney disease. As disease progresses, plasma levels of vitamin D and calcium begin to decline, thus contributing to greater secretion of PTH. In addition, the retention of phosphate further increases PTH secretion independent of calcium and vitamin D levels. PTH has been identified as an important cardiotoxin in HD. Previous studies have supported the view that high PTH serum levels in uremic patients may cause deleterious effects in myocardium metabolism and function (12).
2. AIM
The aim of this study is to investigate the association of β2-M with inflammatory markers, dyslipidemia and mineral disorders in high-flux membrane hemodialysis patients.
3. MATERIALS AND METHODS
In study were included 40 patients, undergoing maintenance high-flux membrane hemodialysis treatment in the Clinical Centre in Prishtina, for a period longer than 6 months. The blood samples were collected between January and May 2013. The criteria for patient’s selection was a high levels of β2-M. Age range were from 24 to 65 year. a) Based in patient’s history, angina, possible myocardial infarction, cerebrovascular events, infective diseases and cancer, were excluded. In all patients, β2-M, CRP, IL-6, triglycerides, cholesterol, LDL, HDL, Lp(a), PTH, Calcium, Phosphorus and serum albumin were determined. Triglycerides, cholesterol, HDL, LDL, Calcium, Phosphorus and albumin were measured by biochemical analysis; b) The assay of β-2 Microglobulin is based on a latex enhanced immunoturbidimetric method. c) CRP was determined by the turbidimetric method, IL-6 with Enzyme Linked Immunosorbent Immunoassay (ELISA), Lipoprotein (a) with immunoturbidimetric method depth with a chemiluminometric immunoassay; d) Twenty-four healthy subjects (12 women and 12 men, aged 56.08 ± 12.34 years) served as controls.
4. RESULTS
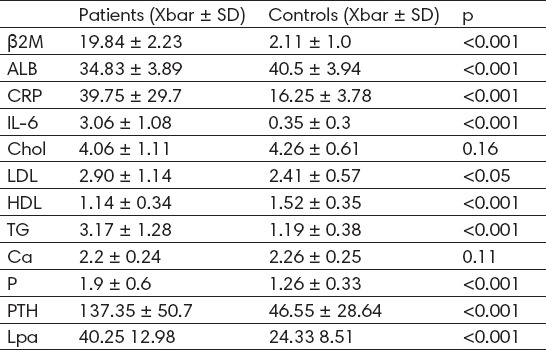
Serum concentration of β2M, CRP, IL-6, triglycerides, Lp(a), P and PTH in hemodialysis patients were significantly higher than in controls (19.84 ± 2.23 mg/L vs 2.11 ± 1.0 mg/L, p<0.001; 39.75± 29.7 mg/L vs 16.25 ± 3.78mg/L, p<0.001; 3.06 ± 1.08 pg/ml vs 0.35 ± 0.3 pg/ml, p < 0.001; 3.17 ± 1.28 mmol/L vs 1.19 ± 0.38 mmol/L, p<0.001; 40.25 ± 12.98 mg/dl vs 24.33 ± 8.51 mg/dL, p<0.001; 1.9 ± 0.6 mmol/L vs 1.26 ± 0.33 mmol/L, p<0.001; 137.35 ± 50.7 pg/ml vs 46.56 ± 28.64 pg/ml, p<0.001 (Table 1). The concentration of HDL and serum albumin was significantly lower (1.14 ± 0.34 vs 1.52 ± 0.35 mmol/L, p<0.001; 34.83 ± 3.89 g/L vs 40.5 ± 3.94 g/L, p<0.001 (Table 1). We did not find any difference in cholesterol, LDL and calcium levels between two groups (4.0 ± 1.11mmol/L vs 4.26 ± 0.16 mmol/L, p=0.16; 2.90 ± 1.14 mmol/L vs 2.41 ± 0.57 mmol/L, p<0.05; 2.2 ± 0.24 mmol/L vs 2.26 ± 0.25 mmol/L, p = 0.11 (Table 1).
Table 1.
Biochemical parameters in hemodialysis patients and controls
β2M was inversely associated with HDL, albumin and calcium concentration (r = -0.73, p <0.001; r =-0.53, p <0.001; r = -0.50, p <0.01 (Table 2), whereas positively was associated with triglycerides, P and PTH (r= 0.69, p <0.001; r= 0.53, p <0.001; r=0.58, p <0.05 (Table 2). The patients with high β2M values, simultaneously had higher Lp(a) concentrations, but we did not observe significant correlation (r=0.28 (Table 2). We also found low positive correlation of β2M with age (r=0.46, p <0.001 (Table 2).
Table 2.
Correlation between serum β2M levels with selected biochemical parameters
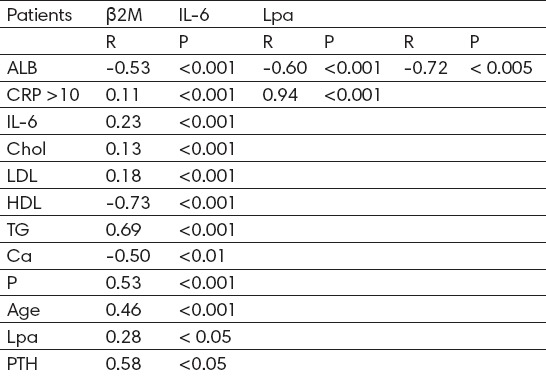
β2M levels were upper in patients with high CRP levels, but there was no significant relationship between CRP or IL-6 and β2M (r= 0.11, p <0.001; r= 0.23, p <0.001 (Table 2). There was no correlation of β2M with cholesterol and LDL cholesterol (r = 0.13, p <0.001; r = 0.18, p <0.001 (Table 2). Positive correlation exists between CRP and IL-6 (r=0.94, p <0.001 (Table 2). Albumins correlated negatively with IL-6 and Lpa (r = -0.60, p <0.001; r=-0.72, p<0.005 (Table 2).
During the follow-up period of three years, 6 out of 40 patients had died, from cardiovascular events.
5. DISCUSSION
β2M is one of cardiovascular risk factors in hemodialysis patients (13). Significant correlation exists between B2M and glomerular filtration rate even when renal function was only slightly impaired. Liabeuf, et al, reported plasma β2M level to be a predictor of cardiovascular events and mortality in patients with different stages of chronic kidney disease (14). One of factors that can affect β2M is the membrane type of hemodialysis. We examined the impact of β2M on some cardiovascular risk factors in high-flux membrane hemodialysis patients. A correlation between an inflammatory response during hemodialysis and elevated serum β2M has been described (15). Compared with the controls, HD patients exhibited marked elevation of serum β2M, CRP and IL-6 (19.84 ± 2.23 mg/L vs 2.11 ± 1.0 mg/L, p<0.001; 39.75 ± 29.7mg/L vs 16.25 ± 3.78mg/L, p<0.001; 3.06 ± 1.08 pg/ml vs 0.35 ± 0.3 pg/ml, p < 0.001 (Table 1). But we found no relationship between CRP or IL-6and β2M (r= 0.11, p <0.001; r= 0.23, p <0.001 (Table 2) when high-flux membranes are used. Perhaps it was because of high β2M levels in all studied patients. The results of our study were similar to the studies of the other authors. (16, 17, 18). Even in studies, where the low flux membranes were used, there was no relations between CRP and β2-M (19).
Our findings suggested that plasma B2M level is directly correlated with some metabolic and cardiovascular risk factors. Triglycerides start to increase in early stages of chronic kidney disease and show the highest values in dialysis patients. Hypertriglyceridemia generates atherogenic small dense LDL particles. Hemodialysis patients presented increased triglycerides compared with controls (3.17 ± 1.28 mmol/L vs 1.19 ± 0.38 mmol/L, p<0.001 (Table 1). We find significant positive correlation between triglycerides and β2M levels (r= 0.69, p <0.001 (Table 2). Reduced HDL level and HDL dysfunction are the hallmarks of hemodialysis, related dyslipidemia. HDL concentrations exhibited significant reduction in patients compared to controls (1.14 ± 0.34 vs 1.52 ± 0.35 mmol/L, p<0.001 (Table 1). Serum β2M concentrations were inversely associated with HDL (r = -0.73, p<0.001 (Table 2). Supporting, linear regression analysis confirmed the negative impact of β2M concentrations on HDL level. Our results are similar with other findings (20, 21). There was no correlation of β2M with cholesterol and LDL.
Lp(a) start increasing early during the course of chronic kidney disease and becomes pronounced with increasing severity of disease. Lp(a) levels were significantly higher in patients than in controls (40.25 ± 12.98 mg/dl vs 24.33 ± 8.51 mg/dL, p<0.001 (Table 1), which confirm that kidney have an important role in Lp(a) metabolism. The patients with high β2M values, simultaneously had higher Lp(a) concentrations but we did not observe high correlation (r=0.28, p<0.05 (Table 2). The negative correlation of Lp(a) with albumin (r=-072, p<0.005 (Table 2), suggests that the mechanism behind the increased Lp(a) levels may be related to the protein losses, perhaps via an increased synthesis rate of apolipoprotein (a) in the liver or via decreased Lp(a) catabolism in HD patients.
Albuminuria and estimated glomerular filtration were multiplicatively associated with all-cause mortality. Similar associations were observed for cardiovascular mortality. We found a significant indirect relationship between β2M and albumin (r=-0.53, p<0.001 (Table 2). This correlation may be used to identify the patients at high risk, from cardiovascular disease (22).
Chronic kidney disease – mineral and bone disorder is a growing health care concern associated with secondary hyperparathyroidism, mineral abnormalities, and increased risk of cardiovascular disease (23).
In hemodialysis patients, concentrations of PTH and P were significantly higher compared with controls (137.35 ± 50.7 pg/ml vs 46.56 ± 28.64 pg/ml, p<0.001; 1.9 ± 0.6 mmol/L vs 1.26 ± 0.33 mmol/L, p<0.001 (Table 1). Calcium levels were lower in patients compare with controls, but with no significant difference (2.2 ± 0.24 mmol/L vs 2.26 ± 0.25 mmol/L, p = 0.11 (Table 1).
Mineral and bone disorders are associated with accelerated atherosclerosis (24), which is an important cause of cardiovascular death in chronic hemodialysis (25). In this study, serum concentration of β2M positively correlated with P and PTH (r= 0.53, p <0.001; r=0.58, p <0.05 (Table 2), whereas negatively with calcium (r = -0.50, p <0.01 (Table 2), which proves that β2M has direct impact in mineral disorders and cardiovascular risk in HD patients. It has been suggested that the predictive value of serum β2M concentration is superior to that provided by established prognostic factors for mortality, such as glomerular filtration, cystatin C and CRP (26). During the follow-up period of three years, 6 out of 40 patients had died from cardiovascular events.
CONCLUSION
Even if there was no correlation of β2M with inflammation, in high-flux membrane hemodialysis patients, our finding indicates that β2M might have an important role in the development of cardiovascular diseases.
Footnotes
• Conflict of interest: none declared.
REFERENCES
- 1.Sameiro Faria M, Ribeiro S, Costa E, et al. Risk Factors for Mortality in Hemodialysis Patients: Two-Year Follow-Up Study. Disease Markers. 2013;35(6):791–8. doi: 10.1155/2013/518945. http://dx.doi.org/10.1155/2013/518945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Himmelfarb J, Stenvinkel P, Ikizler TA, et al. The elephant in uremia: oxidant stress as a unifying concept of cardiovascular disease in uremia. Kidney Int. 2002;62(5):1524–38. doi: 10.1046/j.1523-1755.2002.00600.x. doi:10.1046/j.1523-1755.2002.00600. [DOI] [PubMed] [Google Scholar]
- 3.Locatelli F, Gauly A, Czekalski S, et al. The MPO Study: just a European HEMO Study or something very different? Blood Purif. 2008;26(1):100–4. doi: 10.1159/000110574. [DOI] [PubMed] [Google Scholar]
- 4.Kazama J, Maruyama H, Gejyo F. Reduction of circulating b2-microglobulin level for the treatment of dialysis-related amyloidosis. Nephrol Dial Transplant. 2001;16(4):31–5. doi: 10.1093/ndt/16.suppl_4.31. [DOI] [PubMed] [Google Scholar]
- 5.Wilson AM, Kimura E, Harada RK, et al. B2-microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation. 2007;116(12):1396–1403. doi: 10.1161/CIRCULATIONAHA.106.683722. doi:10.1161/CIRCULATIONAHA.106.683722. [DOI] [PubMed] [Google Scholar]
- 6.Amighi J, Hoke M, Mlekusch W, et al. Beta 2 Microglobulin and the Risk for Cardiovascular Events in Patients With Asymptomatic Carotid Atherosclerosis. Stroke. 2011;42(7):1826–33. doi: 10.1161/STROKEAHA.110.600312. doi:10.1161/STROKEAHA.110.600312. [DOI] [PubMed] [Google Scholar]
- 7.Perez-Garcia R, Rodriguez-Benitez P. Why and how to monitor bacterial contamination of dialysate? Nephrol Dial. Transplant. 2000;15:760–4. doi: 10.1093/ndt/15.6.760. doi:10.1093/ndt/15.6.760. [DOI] [PubMed] [Google Scholar]
- 8.Tsimihodimos V, Mitrogianni Z, Elisaf M. Dyslipidemia Associated with Chronic Kidney Disease. Open Cardiovasc Med J. 2011;5:41–8. doi: 10.2174/1874192401105010041. doi:10.2174/1874192401105010041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jacobson T. Lipoprotein (a), Cardiovascular Disease, and Contemporary Management. Mayo Clinic Proceedings. 2013;88(11):1294–1311. doi: 10.1016/j.mayocp.2013.09.003. doi:10.1016/j.mayocp.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 10.Nordestgaard BG, Chapman MJ, Ray K, et al. Lipoprotein(a) as a cardiovascular risk factor: current status. European Heart Journal. 2010;31:2844–53. doi: 10.1093/eurheartj/ehq386. doi: http://dx.doi.org/10.1093/eurheartj/ehq386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guideline Working Group Japanese Society for Dialysis Therapy. Clinical practice guideline for the management of secondary hyperparathyroidism in chronic dialysis patients. Ther Apher Dial. 2008;12(6):514–25. doi: 10.1111/j.1744-9987.2008.00648.x. doi:10.1111/j.1744-9987.2008.00648.x. [DOI] [PubMed] [Google Scholar]
- 12.Nikodimopoulou M, Liakos S. Secondary hyperparathyroidism and target organs in chronic kidney disease. Hippokratia. 2011;15(1):33–8. [PMC free article] [PubMed] [Google Scholar]
- 13.Zumrutdal A. Role of β2-microglobulin in uremic patients may be greater than originally suspected. World J Nephrol. 2015 Feb 6;4(1):98–104. doi: 10.5527/wjn.v4.i1.98. doi:10.5527/wjn.v4.i1.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liabeuf S, Lenglet A, Desjardins L, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82(12):1297–303. doi: 10.1038/ki.2012.301. [DOI] [PubMed] [Google Scholar]
- 15.Vraetz T, Ittel TH, Mackelenbergh MG. Regulation of B2-microglobulin expression in different human cell lines by proinflammatory cytokines. Nephrol Dial Transplant. 1999;14(9):2137–43. doi: 10.1093/ndt/14.9.2137. [DOI] [PubMed] [Google Scholar]
- 16.Rahbar M, Mehdipour-Aghabagher B. Effect of Inflammatory Factors on β2-Microglobulin in Hemodialysis Patients. Shiraz -E Medical Journal. 2012;13(2):59–62. [Google Scholar]
- 17.Hakim RM, Wingard RL, Husni L, et al. The Effect of Membrane Biocompatibility on Plasma B2-microglobulin Levels in Chronic Hemodialysis Patients. J Am Soc Nephrol. 1996;7(3):472–8. doi: 10.1681/ASN.V73472. [DOI] [PubMed] [Google Scholar]
- 18.Rahbar M, Chitsazan Z, Moslemi B. Correlation between CRP and beta-2 microglobulin in chronic hemodialysis patients with high-flux membrane. Tehran Univ Med J. 2015;73(1):49–54. [Google Scholar]
- 19.Kalocheretis P, Revela I, Spanou E, et al. Strong Correlation of B2-Microglobulin with Procalcitonin in the Serum of Chronic Hemodialysis Patients: A Role for Infections in the Dialysis-Related Amyloidosis? Ren Fail. 2008;30(3):261–5. doi: 10.1080/08860220701857134. [DOI] [PubMed] [Google Scholar]
- 20.Kyriaki D, Kanellopoulos P, Raikou V. High-Density Lipoproteins and Inflammation in Patients on Renal Replacement Therapies. American Journal of Epidemiology and Infectious Disease. 2014;2(1):33–40. doi:10.12691/ajeid-2-1-7. [Google Scholar]
- 21.Kim KM, Kim SS, Kim H, et al. Higher serum beta2-microglobulin levels are associated with better survival in chronic hemodialysis patients: a reverse epidemiology. Clin Nephrol. 2011;75(5):458–65. doi: 10.5414/cnp75458. [DOI] [PubMed] [Google Scholar]
- 22.Okuno S, Ishimura E, Kohno K, et al. Serum beta2-microglobulin level is a significant predictor of mortality in maintenance haemodialysis patients. Nephrol Dial Transplant. 2009;24(2):571–7. doi: 10.1093/ndt/gfn521. [DOI] [PubMed] [Google Scholar]
- 23.Goodman WG, Quarles LD. Development and progression of secondary hyperparathyroidism in chronic kidney disease: lessons from molecular genetics. Kidney Int. 2008;74(3):276–88. doi: 10.1038/sj.ki.5002287. [DOI] [PubMed] [Google Scholar]
- 24.Raggi P, Giachelli C, Bellasi A. Interaction of vascular and bone disease in patients with normal renal function and patients undergoing dialysis. Nature clinical practice. Cardiovasc Med. 2007;4(1):26–33. doi: 10.1038/ncpcardio0725. [DOI] [PubMed] [Google Scholar]
- 25.Shoji T, Maekawa K, Emoto M, et al. Arterial stiffness predicts cardiovascular death independent of arterial thickness in a cohort of hemodialysis patients. Atherosclerosis. 2010;210(1):145–9. doi: 10.1016/j.atherosclerosis.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 26.Shinkai S, Chaves P.H, Fujiwara Y, et al. Beta2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Arch Intern Med. 2008;168(2):200–206. doi: 10.1001/archinternmed.2007.64. [DOI] [PubMed] [Google Scholar]


